禽分枝杆菌亚种的跨种毒力策略。副结核:绵羊和瓜纳科斯的基因表达和感染进展。
IF 2.5
3区 医学
Q2 PARASITOLOGY
引用次数: 0
摘要
致病菌通过调节毒力基因表达来适应宿主。这种灵活和反应性的毒力通常与细菌病原体感染各种宿主物种(包括野生动物和家畜)的能力有关。鸟分枝杆菌亚种副结核(MAP)是约翰氏病的病原体,感染广泛的反刍类食草动物。虽然它在牲畜中的毒性已经得到了充分的研究,但它在野生动物宿主中的行为仍然知之甚少。通过研究感染进程和毒力基因表达,我们对细菌如何引起感染以及宿主如何反应有了重要的认识。在这里,我们检测了从家羊和guanaco (Lama guanicoe)粪便样本中获得的MAP分离株的毒力,包括MAP感染进展和6个MAP基因、2个氧化应激反应基因(katG, sodA)和4个毒力相关基因(impA, umaA1, papA2, kdpC)的表达。使用粪便排出估计作为代理指标来监测感染的进展。与瓜纳瓜相比,绵羊的MAP脱落量和6个基因的表达量均显著增加。这些结果表明,MAP可以根据宿主环境改变其表达谱,也可能是针对宿主特异性的MAP转录调节。这些数据并不排除瓜纳瓜是其自身物种和该地区其他map易感物种的潜在感染源。在混合物种利用景观中,管理跨物种传播和MAP持久性需要了解这些机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
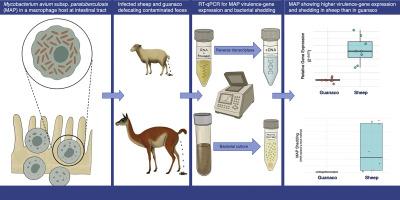
Cross-species virulence strategies of Mycobacterium avium subsp. paratuberculosis: Gene expression and infection progression in sheep and guanacos
Pathogenic bacteria adapt to their hosts modulating virulence-gene expression. This flexible and responsive virulence is frequently associated with the capacity of bacterial pathogens to infect a variety of host species, including wildlife and domestic animals. Mycobacterium avium subsp. paratuberculosis (MAP) is the causative agent of Johne's disease and infects a broad range of ruminant-like herbivores. While its virulence has been well-studied in livestock, its behavior in wildlife hosts remains poorly understood. By studying infection progression and virulence-gene expression, we gain important insights into how bacteria cause infection and how the host reacts. Here, we examine virulence of MAP isolates obtained from fecal samples of domestic sheep and guanaco (Lama guanicoe), including MAP infection progression and expression of six MAP genes, two oxidative stress response genes (katG, sodA) and four virulence-associated genes (impA, umaA1, papA2, kdpC). The progression of the infection was monitored using fecal shedding estimates as a proxy indicator. Compared to guanaco, sheep exhibited both noticeably higher MAP shedding and expression of all six genes. These results indicate that MAP modifies its expression profile in response to the host environment, and also to a possible host-specific transcriptional modulation of MAP. These data did not exclude guanaco as a potential source of infection for both their own species and for other MAP-susceptible species in the area. In mixed species-use landscapes, managing cross-species transmission and MAP persistence requires an understanding of these mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta tropica
医学-寄生虫学
CiteScore
5.40
自引率
11.10%
发文量
383
审稿时长
37 days
期刊介绍:
Acta Tropica, is an international journal on infectious diseases that covers public health sciences and biomedical research with particular emphasis on topics relevant to human and animal health in the tropics and the subtropics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


