百合内生真菌Trochila sp.的生物活性特化代谢产物。
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对一种未被描述的百合内生真菌Trochila sp. BGP15P7IS3的水稻培养物进行了生物检测,分离并鉴定了11种特殊代谢物,其中4种吡啶酮生物碱衍生物(1、2、4和5)和1种联苯衍生物(8)被鉴定为先前未被描述的天然产物。此外,首次从内生真菌中分离到S绝对构型的二苯并呋喃衍生物异音酸和音酸(9和10)。此外,测定了上述化合物7的绝对立体化学性质。通过HR-ESI-MS, 1-和2D NMR, ECD计算以及利用先前发表的数据确定了分离化合物的结构。所有获得的足够数量的分离株都被评估了它们对植物病原体灰霉病菌、四种人类病原体烟曲霉、黄曲霉、白色念珠菌和新型隐球菌以及人类性传播寄生虫阴道毛滴虫的抗菌活性。化合物3仅对新生弧菌具有抑制活性,MIC值为12.5 μg/mL;化合物6对上述真菌病原菌均具有抑制活性,MIC值为0.39 ~ 3.25 μg/mL,对阴道T.具有浓度依赖性的生长抑制作用。在衍生物鉴定的基础上,提出了一种改进的吡酮类生物碱生物合成途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
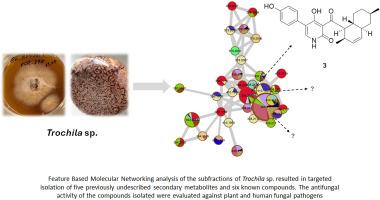
Bioactive specialized metabolites of Trochila sp., an endophytic fungus of Lilium carniolicum
Bioprospecting of a rice culture of Trochila sp. BGP15P7IS3, an undescribed endophytic fungus from Lilium carniolicum, resulted in isolation and identification of eleven specialized metabolites, of which four pyridone alkaloid derivatives (1, 2, 4, and 5) and a biphenyl derivative (8) were identified as previously undescribed natural products. Moreover, two dibenzofuran derivatives, isousnic acid and usnic acid (9 and 10) with S absolute configuration were reported for the first time from an endophytic fungus. Additionally, the absolute stereochemistry of the previously described compound 7 was determined. The structure of the compounds isolated was established by means of HR-ESI-MS, 1- and 2D NMR, and ECD calculations, as well as utilizing previously published data. All isolates available in sufficient quantities were evaluated for their antimicrobial activity against a plant pathogen, Botrytis cinerea, and four human pathogens, Aspergillus fumigatus, Aspergillus flavus, Candida albicans, and Cryptococcus neoformans, as well as a human sextually transmitted parasite, Trichomonas vaginalis. Compound 3 displayed antifungal activity only against C. neoformans with an MIC value of 12.5 μg/mL, and compound 6 exhibited promising antifungal activity with MIC values of 0.39–3.25 μg/mL against all the aforementioned fungal pathogens, and a concentration-dependent growth inhibitory property against T. vaginalis. Based on the identification of derivatives, a revised biosynthetic pathway for pyridone-type alkaloids was proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


