Okhee Yoo, Wenting Li, Siyu Ruan, Elizabeth Syme, Alisha Rodrigo, Connelia Locher, Sharmin Sultana, Lee Yong Lim
下载PDF
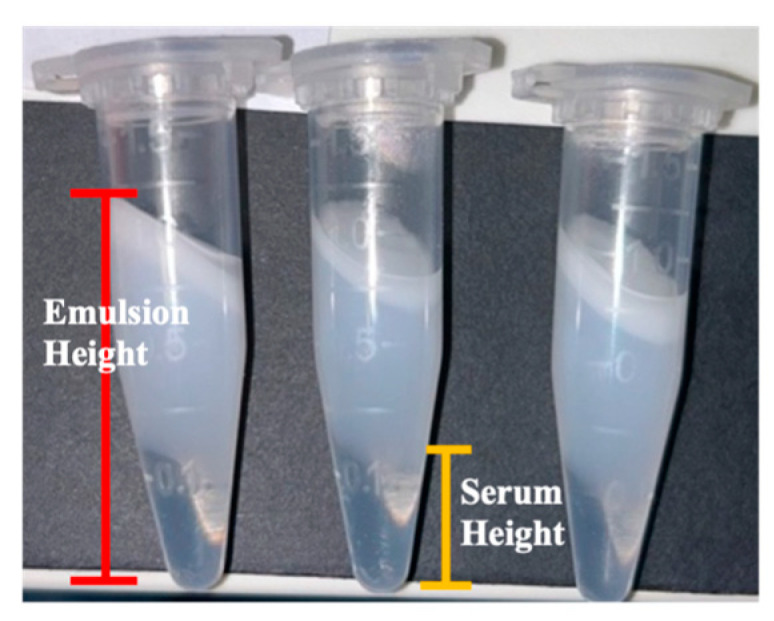
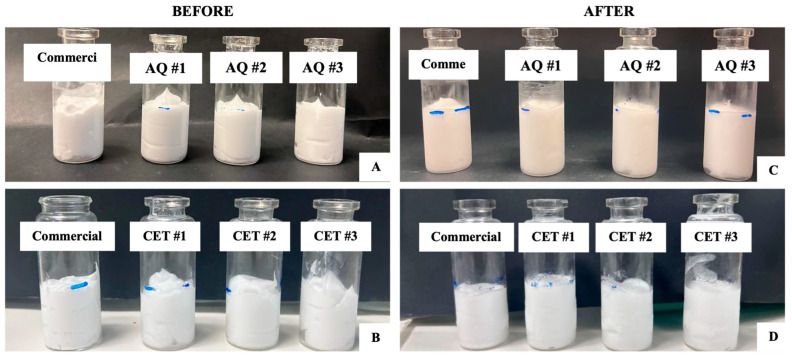
{"title":"用设计方法优化药物配伍质量:两种水乳膏配方的案例研究。","authors":"Okhee Yoo, Wenting Li, Siyu Ruan, Elizabeth Syme, Alisha Rodrigo, Connelia Locher, Sharmin Sultana, Lee Yong Lim","doi":"10.3390/pharmaceutics17091232","DOIUrl":null,"url":null,"abstract":"<p><p><b>Background/Objectives</b>: Quality-by-Design (QbD) is a proactive, risk-based, regulatory-endorsed approach to the development and manufacture of medicinal products but is rarely applied to medicines compounded by pharmacists. This study aims to apply the QbD approach to optimise the compounding processes for the aqueous cream and cetomacrogol cream formulations listed in the Australian Pharmaceutical Formulary and Handbook (APF). <b>Methods</b>: The creams were prepared by varying the process conditions, including oil and water phase temperatures, stirring speed, cooling environment temperature, and the temperature at the end of stirring. Thirty-two samples of each cream type were prepared using combinations of processing conditions defined by a three-level factorial design. The viscosity, spreadability and creaming index of samples were assessed as response variables, and results were analysed using Stat-Ease 360© software to determine the optimal processing conditions for the two creams. To validate the predictive model and assess further cream stability, triplicate creams of each formulation were prepared using the optimised conditions and evaluated for dynamic viscosity, spreadability and creaming index. <b>Results</b>: Optimal conditions for aqueous cream involved heating the oil and water phases to 60 °C and 80 °C, respectively, followed by stirring the two phases at 250 rpm at 10 °C until cooling to 50 °C. For cetomacrogol cream, optimal compounding required heating the oil and water phases to 70 °C and 75 °C, respectively, with stirring the two phases at 220 rpm at ambient temperature (25 °C) until cooling to 40 °C. The conditions predicted by the models successfully yielded creams that met all specified targets. Creams compounded under optimal conditions exhibited well-defined oil droplets, with uniform droplet size in aqueous cream and mild size heterogeneity in cetomacrogol cream. Freeze-thaw testing demonstrated that both optimised creams were stable with no observable phase separation. <b>Conclusions</b>: This study demonstrates that a systematic experimental approach to optimising compounding parameters for the APF aqueous cream and cetomacrogol cream resulted in high-quality, stable, and reproducible products. Formulary guidelines, such as the APF, could benefit from adopting QbD approaches to improve the standardisation of compounding instructions in pharmacy practice.</p>","PeriodicalId":19894,"journal":{"name":"Pharmaceutics","volume":"17 9","pages":""},"PeriodicalIF":5.5000,"publicationDate":"2025-09-22","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12473363/pdf/","citationCount":"0","resultStr":"{\"title\":\"Optimisation of Medicine Compounding Using Quality by Design Approach: Case Studies of Two Aqueous Cream Formulations.\",\"authors\":\"Okhee Yoo, Wenting Li, Siyu Ruan, Elizabeth Syme, Alisha Rodrigo, Connelia Locher, Sharmin Sultana, Lee Yong Lim\",\"doi\":\"10.3390/pharmaceutics17091232\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p><p><b>Background/Objectives</b>: Quality-by-Design (QbD) is a proactive, risk-based, regulatory-endorsed approach to the development and manufacture of medicinal products but is rarely applied to medicines compounded by pharmacists. This study aims to apply the QbD approach to optimise the compounding processes for the aqueous cream and cetomacrogol cream formulations listed in the Australian Pharmaceutical Formulary and Handbook (APF). <b>Methods</b>: The creams were prepared by varying the process conditions, including oil and water phase temperatures, stirring speed, cooling environment temperature, and the temperature at the end of stirring. Thirty-two samples of each cream type were prepared using combinations of processing conditions defined by a three-level factorial design. The viscosity, spreadability and creaming index of samples were assessed as response variables, and results were analysed using Stat-Ease 360© software to determine the optimal processing conditions for the two creams. To validate the predictive model and assess further cream stability, triplicate creams of each formulation were prepared using the optimised conditions and evaluated for dynamic viscosity, spreadability and creaming index. <b>Results</b>: Optimal conditions for aqueous cream involved heating the oil and water phases to 60 °C and 80 °C, respectively, followed by stirring the two phases at 250 rpm at 10 °C until cooling to 50 °C. For cetomacrogol cream, optimal compounding required heating the oil and water phases to 70 °C and 75 °C, respectively, with stirring the two phases at 220 rpm at ambient temperature (25 °C) until cooling to 40 °C. The conditions predicted by the models successfully yielded creams that met all specified targets. Creams compounded under optimal conditions exhibited well-defined oil droplets, with uniform droplet size in aqueous cream and mild size heterogeneity in cetomacrogol cream. Freeze-thaw testing demonstrated that both optimised creams were stable with no observable phase separation. <b>Conclusions</b>: This study demonstrates that a systematic experimental approach to optimising compounding parameters for the APF aqueous cream and cetomacrogol cream resulted in high-quality, stable, and reproducible products. Formulary guidelines, such as the APF, could benefit from adopting QbD approaches to improve the standardisation of compounding instructions in pharmacy practice.</p>\",\"PeriodicalId\":19894,\"journal\":{\"name\":\"Pharmaceutics\",\"volume\":\"17 9\",\"pages\":\"\"},\"PeriodicalIF\":5.5000,\"publicationDate\":\"2025-09-22\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12473363/pdf/\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"Pharmaceutics\",\"FirstCategoryId\":\"3\",\"ListUrlMain\":\"https://doi.org/10.3390/pharmaceutics17091232\",\"RegionNum\":3,\"RegionCategory\":\"医学\",\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"\",\"PubModel\":\"\",\"JCR\":\"Q1\",\"JCRName\":\"PHARMACOLOGY & PHARMACY\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"Pharmaceutics","FirstCategoryId":"3","ListUrlMain":"https://doi.org/10.3390/pharmaceutics17091232","RegionNum":3,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"PHARMACOLOGY & PHARMACY","Score":null,"Total":0}
引用次数: 0
引用
批量引用


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


