肺移植后早期成人受体中霉酚酸的人群药代动力学及贝叶斯估计。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
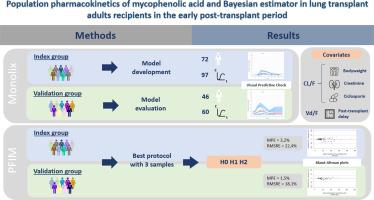
霉酚酸(MPA)作为其前药霉酚酸酯(Mycophenolate mofetil, MMF)被广泛用于预防器官实体移植后的排斥反应。MPA在肺移植中的群体药代动力学研究很少,因此本研究旨在描述MPA的群体药代动力学(PK),并建立移植后早期肺移植(LT)受者MPA曲线下面积(AUC0-12h)的最大后验贝叶斯估计量。我们在普莱西-罗宾逊医院(法国)进行了一项单中心回顾性研究,包括在移植后的前3个月接受MMF的肝移植受体,其中至少有一种浓度-时间谱。分别于给药前及给药后0.5、1、2、4±6 h测定MPA。患者分为指标组(N=72)和验证组(N=46)。利用索引数据集建立了种群PK模型和三次采样的贝叶斯估计器,并利用验证数据集进行了外部评估。使用Monolix®软件进行非线性混合效应模型分析。利用指数数据集中的97条MPA剖面建立了PK模型。MPA PK最好用具有滞后时间和一阶吸收消除的双室模型来描述。影响MPA表观清除率的显著协变量为肌酐、体重和环孢素联合给药以及移植后时间对中央室表观容积的影响。该模型成功地在验证数据集中的60个MPA剖面中进行了评估。最佳贝叶斯估计包括服药前、服药后1小时和4小时的样本。在两个数据集中,AUC的预测是无偏的,精度在20%左右。这是第一个可以预测移植后早期无囊性纤维化的LT患者AUC的贝叶斯估计量,为改善MPA的治疗药物监测提供了工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Population pharmacokinetics of mycophenolic acid and Bayesian estimator in lung transplant adults recipients in the early post-transplant period
Mycophenolic acid (MPA), administered as its prodrug Mycophenolate mofetil (MMF), is widely used to prevent rejection after organ solid transplantation. Few population pharmacokinetic studies of MPA in lung transplantation are available, so the present study was designed to describe the population pharmacokinetics (PK) of MPA and develop a maximum a posteriori Bayesian estimator of MPA area under the curve (AUC0-12h) in lung transplant (LT) recipients in the early post-transplant period.
We conducted a single-center retrospective study in the Plessis-Robinson hospital (France), including LT recipients receiving MMF in whom at least one concentration-time profile was available during the first 3 months post-transplantation. MPA was measured before intake and 0.5, 1, 2, 4 ± 6 hours post-administration. Patients were divided into an index group (N=72) and a validation group (N=46). A population PK model and a Bayesian estimator with three sampling times were built with the index dataset and externally evaluated with the validation dataset. Analyses were performed using nonlinear mixed-effects models with Monolix® software.
The PK model was built using 97 MPA profiles in the index dataset. MPA PK was best described using a two-compartment model with a lag time and first-order absorption and elimination. The significant covariates on the MPA apparent clearance were creatinine, body weight and ciclosporin co-administration and the posttransplantation time on the apparent volume of the central compartment. The model was successfully evaluated in the 60 MPA profiles from the validation dataset. The best Bayesian estimator included samples just before intake, 1 and 4 hours post-administration. The prediction of AUC was unbiased in both datasets and had a precision around 20 %. This is the first Bayesian estimator allowing the prediction of AUC in LT patients without cystic fibrosis in the early post-transplant period, providing a tool to improve the therapeutic drug monitoring of MPA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


