MoOx/SiO2催化剂上氧阴离子自由基与Mo(VI)配合物及其在甲烷氧化制甲醇中的作用
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
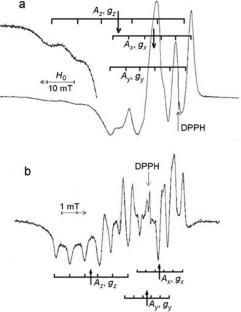
在富含95Mo同位素的MoOx/SiO2样品中发现了与1 Mo(VI)配位的氧阴离子自由基\({O}_{2}^{-\cdot }\)。结果表明,未配对电子主要集中在氧离子上(gz = 2.017, gy = 2.004, gx = 1.999; Ax(95Mo) = 6.1, Ay(95Mo) = 6.0, Az(95Mo)=9.1 G;∆Hx =∆Hy = 2,2 G,∆Hz = 2,6 G)。利用一种特殊的冷冻技术,鉴定了甲基自由基与分子氧在气相中反应产生的过氧化自由基(ch300·)。讨论了Mo(VI)直接配位的氧离子自由基参与甲烷氧化生成甲醇的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oxygen Anion-Radical Complexes with Mo(VI) on MoOx/SiO2 Catalysts and Their Role in the Oxidation of Methane to Methanol
Oxygen anion radicals \({O}_{2}^{-\cdot }\) coordinated with one Mo(VI) have been identified in MoOx/SiO2 samples enriched with 95Mo isotope. It is shown that unpaired electron is mainly localized on the oxygen ion (gz = 2.017, gy = 2.004, gx = 1.999; Ax(95Mo) = 6.1, Ay(95Mo) = 6.0, Az(95Mo)=9.1 G; ∆Hx = ∆Hy = 2,2 G and ∆Hz = 2,6 G). Using a special freezing technique, peroxide radicals (CH3OO·), which are the products of the reaction of the methyl radical with molecular oxygen in the gas phase, have been identified. The oxidation pathways of methane to methanol with participation of the oxygen ion radicals coordinated directly by Mo(VI) are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


