沙棘果实中具有保肝活性的结构多样性黄酮木脂素
IF 0.9
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
首次从沙棘中分离到一种新的黄酮木聚糖,命名为3,3 ',4 ',5 ' -四羟基-3 ',5 ' -二甲氧基-9 ',9 ‘ ’二羟甲基-4 ' -羟基-3 ',5 ' ' -二甲氧基苯基黄酮木聚糖(1),以及已知的5种黄酮木聚糖衍生物(2-6)。通过广泛而全面的红外、紫外、核磁共振、质谱及文献对其结构进行了鉴定。化合物1-6对apap处理的HepG2细胞的肝保护活性进行了评价。结果表明,化合物1和2具有中等程度的肝保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
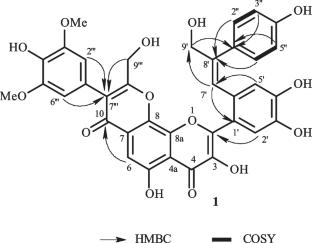
Structurally Diverse Flavonolignans with Hepatoprotective Activities from the Fruits of Hippophae rhamnoides
A novel flavonolignan, named 3,3′,4′,5-tetrahydroxy-3′′′,5′′′-dimethoxy-9′,9′′′dihydroxymethyl-4- hydroxyphenyl-4′′′-hydroxy-3′′′,5′′′-dimethoxyphenylflavonolignan (1), together with five known flavonolignan derivatives (2–6) were isolated from Hippophae rhamnoides L. for the first time. Their structures were determined by extensive and comprehensive IR, UV, NMR, MS spectra, as well as reported references. Compounds 1–6 were evaluated for their hepatoprotective activities on APAP-treated HepG2 cells. As a result, compounds 1 and 2 exhibited moderate hepatoprotective activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


