20-羟基蜕皮酮和后睾酮2-酯和二氧烷衍生物的化学选择性合成
IF 0.9
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
提出了以5-溴戊酸、硫辛酸和芳香杂环醛为原料合成甾体酯类和环缩醛类衍生物的化学选择性方法。2-单酯衍生物(化合物4-7)或二恶烷加合物(化合物8-10)的化学选择性形成发生在蜕皮甾体与酸或醛的缩合过程中。化合物8是由20-羟基蜕皮酮与糠醛酸催化缩合而成,其类似物是从牛膝草中分离得到的植物蜕皮甾体serfurrosterone A。本文章由计算机程序翻译,如有差异,请以英文原文为准。
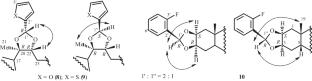
Chemoselective Synthesis of 2-Ester and Dioxolane Derivatives of 20-Hydroxyecdysone and Poststerone
Chemoselective approaches to the synthesis of ester and cyclic acetal derivatives of ecdysteroids with 5-bromovaleric and lipoic acids and aromatic heterocyclic aldehydes were proposed. Chemoselective formation of 2-monoester derivatives (compounds 4–7) or dioxolane adducts (compounds 8–10) occurred during condensation of an ecdysteroid with an acid or aldehyde. Compound 8, which was prepared by acid-catalyzed condensation of 20-hydroxyecdysone with furfuraldehyde, was an analog of the phytoecdysteroid serfurosterone A, which was isolated from the plant Achyranthes bidentata.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


