Fe(II) -Fe (III) -L-Ala-H2O体系络合物形成过程的研究
IF 0.5
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
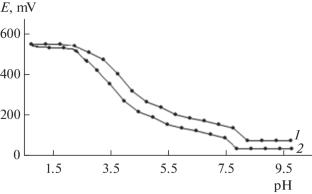
用经典的Clark-Nikolsky氧化电位方法研究了298.15 K下Fe(II) -Fe (III) -L-Ala-H2O体系中配位化合物的形成过程和1.0 mol/L Na(H)ClO4背景下溶液的离子强度。通过对所得到的体系电动势随基本粒子浓度参数变化的实验曲线的联合分析,可以确定下列配合物的形成:[FeHL(H2O)5]3+, [Fe(HL)2(H2O)4]3+, [Fe2(HL)2(OH)4(H2O)6]2+, [Fe(III)Fe(II)(HL)2(OH)4(H2O)6]+, [FeHL(H2O)5]2+, [Fe(HL)(OH) 4]+,和[Fe(HL)(OH)2(H2O)3]0。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Study of the Processes of Complex Formation in a Fe(II)–Fe(III)–L-Ala–H2O System
The processes of the formation of coordination compounds in a Fe(II)–Fe(III)–L-Ala–H2O system at 298.15 K and the ionic strength of a solution of 1.0 mol/L against the background of Na(H)ClO4 are studied by the classical method of the Clark–Nikolsky oxidation potential. A joint analysis of the obtained experimental curves of the dependence of the electromotive force of the system on the concentration parameters of basic particles makes it possible to determine the formation of complexes with the following compositions: [FeHL(H2O)5]3+, [Fe(HL)2(H2O)4]3+, [Fe2(HL)2(OH)4(H2O)6]2+, [Fe(III)Fe(II)(HL)2(OH)4(H2O)6]+, [FeHL(H2O)5]2+, [Fe(HL)(OH)(H2O)4]+, and [Fe(HL)(OH)2(H2O)3]0.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Moscow University Chemistry Bulletin
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.30
自引率
14.30%
发文量
38
期刊介绍:
Moscow University Chemistry Bulletin is a journal that publishes review articles, original research articles, and short communications on various areas of basic and applied research in chemistry, including medical chemistry and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


