用腙基团功能化的苯并二恶烯:合成、偶氮腙互变异构和酸碱平衡
IF 0.8
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以2,5-二叔丁基-3-羟基-1,4-苯醌与芳基肼为原料,合成了具有腙基的新型苯二氧羰基衍生物。偶氮腙的原生互变异构性取决于腙片段中芳基取代基的性质。此外,合成的化合物对介质的酸度敏感,其敏感程度由腙段芳基取代基的受体性质调节。具有2,4-二硝基苯取代基的腙具有最高的灵敏度和良好的可逆性,显示了其在非水介质中作为酸碱指示剂的潜在用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
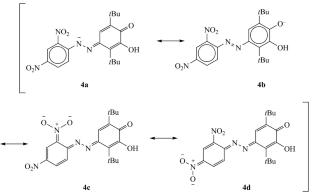
Benzodioxolenes Functionalized with Hydrazone Moiety: Synthesis, Azo-Hydrazone Tautomerism, and Acid-Base Equilibrium
Novel benzodioxolene derivatives with hydrazone moiety were obtained by the reaction of 2,5-di-tert-butyl-3-hydroxy-1,4-benzoquinone with arylhydrazines. It was found that azo-hydrazone prototropic tautomerism depends on the nature of aryl substituent in hydrazone fragment. Moreover, synthesized compounds are sensitive to the acidity of the medium, and the degree of sensitivity is modulated by the acceptor properties of the aryl substituent in hydrazone moiety. The highest sensitivity combined with excellent reversibility was found for hydrazone with a 2,4-dinitrophenyl substituent, showing its potential use as an acid-base indicator in non-aqueous media.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


