用于去除硫化氢气体的低成本生物质灰基吸附剂
IF 7.5
3区 环境科学与生态学
Q2 ENERGY & FUELS
引用次数: 0
摘要
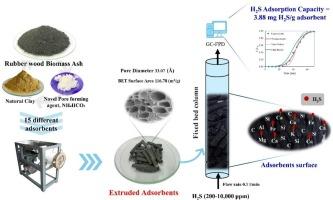
本研究旨在利用锅炉-生物质灰制备低成本高效的吸附剂,用于去除小型沼气厂沼气中的硫化氢。为了减少实际吸附过程中吸附床间的压降,选择粘土作为粘结剂进行挤压吸附剂。以不同灰土比(70 ~ 90%)和NH4HCO3(0 ~ 6%)为成孔剂,焙烧温度(100 ~ 500℃)为条件,制备了15种不同的吸附剂。通过0.1 L/min含合成H2S气体进料固定床柱的突破性固化研究,发现R2为0.75的二次方程可用于优化吸附制备和预测H2S吸附量。以79%灰分、2.5% NH4HCO3为吸附剂,在293℃条件下制备的最佳吸附剂吸附H2S的容量为3.67 ~ 3.88 mg/g。表征结果,包括BET表面积分析和SEM成像,表明NH4HCO3的存在导致了明显的孔隙形成。CHNS/O SEM-EDX和XRF分析证实,后吸附剂的硫含量有所增加。废吸附剂的表面积减小,FTIR光谱中官能团的变化,以及支持化学吸附机理的金属元素的存在。对Thomas模型来说,在200-10,000 ppm含H2S气体条件下的最佳拟合突破曲线可以进一步扩大规模。这是一次成功的尝试,利用丰富的工业废物、环保的当地材料和新型的成孔剂,来创造一种生态高效的H2S去除吸附剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Low-cost biomass ash-based adsorbent for removal of hydrogen sulfide gas
This research aims to use boiler-biomass ash to produce low-cost and efficient adsorbent for removing hydrogen sulfide (H2S) in biogas from small scale biogas plant. To reduce pressure drop across adsorbent bed during the practical adsorption, clay was selected as a binder to perform the extruded adsorbent. 15 different adsorbents using different proportion of ash to clay (70–90 %) together with the an amount of NH4HCO3 as pore-forming reagent (0–6 %), and baking temperature (100–500 °C) were prepared. From the breakthrough cure studies using fixed-bed column fed by 0.1 L/min synthetic H2S containing gas, it was found that quadratic equation with R2 0.75 can be used to optimize adsorption preparation and predict H2S adsorption capacity. The optimized adsorbent prepared using 79 % ash, and 2.5 % NH4HCO3 at 293 °C was validated and had achieved H2S adsorption capacity of 3.67–3.88 mg/g. The characterization results, including BET surface area analysis and SEM imaging, show significant pore formation due to the presence of NH4HCO3. CHNS/O SEM-EDX, and XRF analyses confirmed that there wasan increase in sulfur content of the post-adsorbent. The decrease in surface area and change of functional groups in FTIR spectrum of the spent adsorbent, and presence of metal elements supporting the chemisorption mechanism were also discovered. The best fitted breakthrough curve operated at 200–10,000 ppm H2S containing gas to Thomas model which could be implemented for further scaling up are also proposed. This is a successful attempt to use an abundance industrial waste, eco-friendly local material and novel pore forming agent in order to create an eco-efficient adsorbent for H2S removal.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbon Resources Conversion
Materials Science-Materials Science (miscellaneous)
CiteScore
9.90
自引率
11.70%
发文量
36
审稿时长
10 weeks
期刊介绍:
Carbon Resources Conversion (CRC) publishes fundamental studies and industrial developments regarding relevant technologies aiming for the clean, efficient, value-added, and low-carbon utilization of carbon-containing resources as fuel for energy and as feedstock for materials or chemicals from, for example, fossil fuels, biomass, syngas, CO2, hydrocarbons, and organic wastes via physical, thermal, chemical, biological, and other technical methods. CRC also publishes scientific and engineering studies on resource characterization and pretreatment, carbon material innovation and production, clean technologies related to carbon resource conversion and utilization, and various process-supporting technologies, including on-line or off-line measurement and monitoring, modeling, simulations focused on safe and efficient process operation and control, and process and equipment optimization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


