一种新型4-乙烯基吡啶改性净水聚合物:PVP-g-HEMA作为高效溴酸盐吸附剂
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
溴酸盐(BrO₃⁻)是水消毒的一种致癌副产品,对饮用水安全构成重大威胁。本研究将4-乙烯基吡啶接枝到甲基丙烯酸2-羟乙酯上,合成了一种新型高分子吸附剂PVP-g-HEMA。表征证实了其官能团和无定形结构。在pH值为4的条件下,该吸附剂在20 min内达到了95.2%的溴酸盐去除效率。吸附遵循Langmuir等温线(qmax = 0.36 mg/g),动力学模型表明存在物理和化学相互作用。热力学分析表明这是一个自发的放热过程。该聚合物具有良好的可重复使用性,在1 M NaOH条件下,其解吸效率为97.94%。确保溴酸盐的有效去除有助于实现安全管理的饮用水服务。这些结果表明,PVP-g-HEMA作为一种有效的、可再生的吸附剂,可以快速去除水中的溴酸盐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
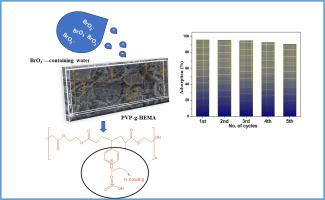
A new 4-vinylpyridine-modified polymer for clean water: PVP-g-HEMA as an efficient bromate adsorbent
Bromate (BrO₃⁻) is a carcinogenic by-product of water disinfection that poses significant risks to drinking water safety. In this study, a novel polymeric adsorbent (PVP-g-HEMA) was synthesized by grafting 4-vinylpyridine onto 2-hydroxyethyl methacrylate. Characterization confirmed its functional groups and amorphous structure. The adsorbent achieved a maximum bromate removal efficiency of 95.2 % at pH 4 within just 20 min. Adsorption followed the Langmuir isotherm (qmax = 0.36 mg/g), and kinetic modeling suggested both physical and chemical interactions. Thermodynamic analysis indicated a spontaneous and exothermic process. The polymer also showed excellent reusability, with 97.94 % desorption efficiency using 1 M NaOH. Ensuring the effective removal of bromate supports efforts toward safely managed drinking water services. These results demonstrate the potential of PVP-g-HEMA as an effective and regenerable adsorbent for rapid bromate removal from water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


