香樟精油通过(+)-2-龙脑酮调节NOX4对乳腺癌的抗肿瘤作用
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景乳腺癌(BC)是女性中最常见的恶性肿瘤。香樟精油(Cinnamomum camphora精油,CCEO)在癌症治疗中已显示出潜力,但其抗肿瘤成分及其在BC中的作用机制尚不清楚。NADPH氧化酶4 (NOX4)是活性氧(ROS)的重要产生者,可推动癌症进展并作为潜在的治疗靶点。目的研究CCEO的抗肿瘤活性,鉴定其活性成分,阐明其分子机制。方法采用MTT法、western blot法和流式细胞术检测CCEO对BC细胞株的抗肿瘤作用。通过western blot和RT-qPCR分析信号通路的调控。采用异种移植肿瘤模型评价其在体内的有效性和安全性。采用气相色谱-质谱法(GC-MS)鉴定CCEO的主要成分,并通过NOX4过表达实验探讨其分子机制。结果scceo通过诱导细胞凋亡和衰老,降低细胞内ROS水平,抑制迁移和侵袭,引起细胞周期阻滞,在体外和体内均表现出明显的抗肿瘤活性。GC-MS分析发现(+)-2-Bornanone是主要的抗肿瘤活性成分。机制上,(+)-2-Bornanone抑制NOX4的表达,从而抑制丝裂原活化蛋白激酶/细胞外信号调节激酶(MAPK/ERK)信号通路,最终抑制肿瘤进展。结论CCEO在三阴性乳腺癌(TNBC)中的抗肿瘤作用主要通过(+)-2-Bornanone介导,通过下调NOX4抑制MAPK/ERK通路。这些发现揭示了一种新的抗肿瘤机制,为进一步开发(+)-2-Bornanone作为治疗BC的天然药物提供了强有力的理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
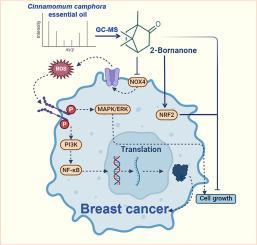
Anti-tumor effects of Cinnamomum camphora essential oil in breast cancer via NOX4 modulation by (+)-2-bornanone
Background
Breast cancer (BC) is the most prevalent malignancy among women. Cinnamomum camphora essential oil (CCEO) has shown potential in cancer therapy, but its anti-tumor components and mechanisms in BC remain unclear. NADPH oxidase 4 (NOX4) is an important producer of reactive oxygen species (ROS), driving cancer progression and serving as a potential therapeutic target.
Purpose
To investigate the anti-tumor activity of CCEO, characterize its active compounds, and clarify its molecular mechanisms.
Methods
The anti-tumor effects of CCEO were assessed in BC cell lines via MTT assays, western blot, and flow cytometry. The modulation of signaling pathways was analyzed via western blot and RT-qPCR. Xenograft tumor models were employed to evaluate efficacy and safety in vivo. Gas chromatography-mass spectrometry (GC–MS) was used to identify major constituents of CCEO, and NOX4 overexpression assays were conducted to explore molecular mechanisms.
Results
CCEO exhibited significant anti-tumor activity both in vitro and in vivo by inducing apoptosis and senescence, decreasing intracellular ROS levels, inhibiting migration and invasion, and causing cell cycle arrest. GC–MS analysis identified (+)-2-Bornanone as the predominant active component responsible for anti-tumor effects. Mechanistically, (+)-2-Bornanone suppressed NOX4 expression, which inhibited the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway, ultimately suppressing tumor progression.
Conclusion
The anti-tumor effects of CCEO in triple-negative breast cancer (TNBC) are principally mediated by (+)-2-Bornanone, which inhibits the MAPK/ERK pathway via NOX4 downregulation. These findings reveal a novel anti-tumor mechanism and provide a strong rationale for further developing (+)-2-Bornanone as a promising natural therapeutic agent for BC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


