肠道微生物代谢物尿素B通过FGFR3/NCOA4/FTH1轴改写铁稳态,抑制软骨细胞铁凋亡,缓解骨关节炎
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
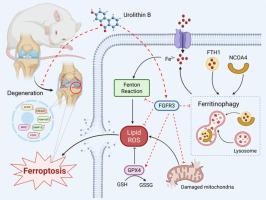
骨关节炎(OA)是一种以关节软骨变性为特征的常见慢性疾病。缺乏安全有效的治疗方法使OA成为全球致残的主要原因,严重影响患者的生活质量。尿素B (UB)是肠道微生物处理鞣花酸产生的一种生物活性代谢物,具有有效的抗氧化和抗炎特性,是一种有前景的OA治疗候选药物。然而,UB抑制OA进展的确切机制尚不清楚。目的本研究旨在评估UB治疗OA的潜力并阐明其潜在的作用机制。方法采用白细胞介素-1β (IL-1β)诱导的软骨细胞模型和前交叉韧带横断(ACLT)大鼠观察UB对骨性关节炎的治疗作用。采用整合转录组分析全面探讨相关机制。在体外利用成纤维细胞生长因子受体3 (FGFR3)特异性小干扰RNA (siRNA)和过表达质粒来验证FGFR3作为UB特异性下游靶点的作用及其在骨关节炎进展中的功能意义。此外,在UB治疗的ACLT大鼠中进行了FGFR3基因敲除,以确认UB及其下游靶点FGFR3的关键作用。本研究报告了一项新发现,UB显著减轻il -1β诱导的细胞外基质降解,并通过一种先前未被认识的涉及铁稳态调节的机制缓解OA的进展。在机制上,我们证明UB通过上调FGFR3表达来抑制软骨细胞铁凋亡,FGFR3通过破坏核受体辅助激活因子4 (NCOA4)和铁蛋白重链1 (FTH1)之间的相互作用来抑制铁蛋白自噬。我们的研究结果确立了FGFR3/NCOA4/FTH1信号轴在炎症条件下通过恢复铁稳态在软骨细胞中的新保护作用。我们发现FGFR3是预防和缓解OA的一个新的治疗靶点。UB作为FGFR3的功能性激动剂的验证,揭示了其作为一种新的候选治疗药物的潜力,并为治疗OA提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Gut microbiota metabolite Urolithin B inhibits chondrocyte ferroptosis by rewriting iron homeostasis via FGFR3/NCOA4/FTH1 axis, alleviating osteoarthritis
Background
Osteoarthritis (OA) is a prevalent chronic disease characterized by articular cartilage degeneration. The lack of safe and effective therapies has made OA a leading global cause of disability, severely impacting patient quality of life. Urolithin B (UB), a bioactive metabolite derived from gut microbiota processing of ellagic acid, exhibits potent antioxidant and anti-inflammatory properties, positioning it as a promising therapeutic candidate for OA. However, the precise mechanisms by which UB inhibits OA progression remain unknown.
Purpose
This study aimed to evaluate the therapeutic potential of UB for OA and elucidate its underlying mechanisms of action.
Methods
The therapeutic effects of UB on OA were assessed using interleukin-1β (IL-1β)-induced chondrocyte models and rats subjected to anterior cruciate ligament transection (ACLT). Integrated transcriptome analysis was employed to comprehensively investigate the relevant mechanisms. Fibroblast growth factor receptor 3 (FGFR3)-specific small interfering RNA (siRNA) and overexpression plasmids were utilized in vitro to validate the role of FGFR3 as a specific downstream target of UB and its functional significance in osteoarthritis progression. Furthermore, FGFR3 gene knockdown was performed in UB-treated ACLT rats to confirm the critical role of both UB and its downstream target, FGFR3.
Results
This study reports the novel discovery that UB significantly mitigates IL-1β-induced extracellular matrix degradation and alleviates OA progression through a previously unrecognized mechanism involving iron homeostasis regulation. Mechanistically, we demonstrate that UB inhibits chondrocyte ferroptosis by upregulating FGFR3 expression, which suppresses ferritinophagy by disrupting the interaction between nuclear receptor coactivator 4 (NCOA4) and ferritin heavy chain 1 (FTH1). Our findings establish the novel protective role of the FGFR3/NCOA4/FTH1 signaling axis in chondrocytes under inflammatory conditions by restoring iron homeostasis.
Conclusions
We identified FGFR3 as a novel therapeutic target for the prevention and alleviation of OA. The validation of UB as a functional agonist of FGFR3, revealing its potential as a new therapeutic candidate and providing a new perspective for combating OA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


