铁和铁碳化物表面依赖的CO2和CO活化:来自密度泛函理论的见解
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通常,铁催化剂会产生各种原位碳化物,这些原位碳化物有时被认为是CO2和CO加氢、逆水气变换(RWGS)和费托合成(FTS)反应中的活性相。然而,关键问题出现在铁碳化物表面铁和碳原子对CO2和CO活化的作用。对η-Fe2C、θ-Fe3C和χ-Fe5C2碳化铁的Fe金属端和Fe- c端表面进行了自旋极化密度泛函理论(DFT)计算,结果表明:(1)相对于Fe端碳化铁和金属铁,表面碳对Fe- c端表面的稳定性更强;(2)相对于Fe- c端碳化铁和金属铁,Fe端碳化铁和金属铁更有利于CO2、CO的放热吸附。此外,电子结构分析表明,由于d带中心离费米能级很远,fe - c端表面的反应性相对较弱。此外,中等稳定的Fe(110)是最优选的表面,与所有考虑的碳化物相比,它有利于CO2和CO的动力学和热力学直接解离。总体而言,本研究系统地分析了表面能、终止态、表面Fe/C比、表面碳化铁中C原子对CO2和CO活化的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
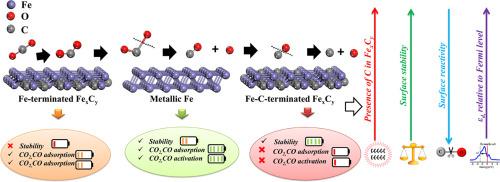
Surface-dependent CO2 and CO activation on iron and iron carbides: Insights from density functional theory
Conventionally, iron catalysts produce a variety of in-situ carbides, which are sometimes identified as the active phase in CO2 and CO hydrogenation, Reverse water gas shift (RWGS) and Fischer–Tropsch synthesis (FTS) reactions. However, the key questions arise towards the role of surface Fe and C atoms of iron carbides towards CO2 and CO activation. Spin-polarised Density Functional Theory (DFT) calculations performed on Fe metal and Fe-C-terminated surfaces of η-Fe2C, θ-Fe3C, and χ-Fe5C2 iron carbides reveal that, (i) surface carbon brings more stability to Fe-C-terminated surfaces compared to Fe-terminated iron carbides and metallic iron, (ii) Fe-terminated iron carbides and metallic iron are more conducive towards the exothermic CO2, CO adsorption than the Fe-C-terminated iron carbides. Moreover, electronic structure analysis unveils that Fe-C-terminated surfaces are comparatively less reactive due to the occurrence of the d-band center very far from the Fermi Level. Furthermore, moderately stable Fe(110) is found as the most preferred surface, favouring direct dissociation of both CO2 and CO kinetically and thermodynamically compared to all carbides considered. Overall, this study systematically analysed the role of surface energy, termination, surface Fe/C ratio, and surface C atom in iron carbides on the activation of CO2 and CO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


