双金属-硼体系:一种很有前途的CO2还原反应电催化剂
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
金属硼化物由于其出色的催化能力,在破坏小分子的惰性化学键方面得到了广泛的应用。同时,双金属催化剂因其活性位点多、化学性质可调而备受关注。受这些发现的启发,通过将两个过渡金属原子(TMs)引入掺杂硼的石墨烯(BG)中,构建了55种不同的双金属-硼基体系。采用密度泛函理论计算研究了它们对CO2还原反应(CO2RR)的催化性能。研究发现MnZn@BG电催化剂在-0.29 V的低极限电位下,能有效地将CO2还原为CH4。在酸性条件下,MnZn@BG的催化性能得到增强。此外,在MnZn@BG上有效抑制了竞争性析氢反应,有助于其对CO2RR的高选择性。机器学习分析表明,除了广泛使用的d波段中心描述子外,TMs上d电子的数量显著影响TM1TM2@BG上的CO2吸附强度。这些结果表明MnZn@BG是一种很有前途的CO2RR电催化剂,为双金属-硼体系在可持续能量转换中的应用提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
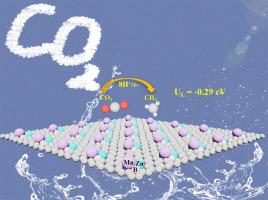
Dual-metal-boron system: A promising candidate electrocatalyst towards CO2 reduction reaction
Metal borides have found extensive use in breaking the inert chemical bonds in small molecules due to their outstanding catalytic capabilities. Meanwhile, the dual-metal catalysts (DACs) have garnered much attention owing to their numerous active sites and adjustable chemical properties. Inspired by these findings, 55 different dual-metal-boron based systems were constructed by introducing two transition metal atoms (TMs) into boron-doped graphene (BG). Their catalytic performance towards CO2 reduction reaction (CO2RR) was studied by employing density functional theory calculations. It is found that the MnZn@BG electrocatalyst could effectively reduce the CO2 into CH4 with a low limiting potential of -0.29 V. The catalytic performance of MnZn@BG is enhanced under acidic conditions. Furthermore, the competitive hydrogen evolution reaction is effectively suppressed on MnZn@BG, contributing to its high selectivity for CO2RR. Machine learning analysis revealed that, except for the widely used d-band center descriptor, the number of d-electrons on TMs significantly influences the CO2 adsorption strength on TM1TM2@BG. These results suggest that the MnZn@BG is a promising candidate as a CO2RR electrocatalyst, offering insights into the application of dual-metal-boron systems in sustainable energy conversion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


