探讨依西美坦负载纳米颗粒在乳腺癌靶向治疗中的疗效和机制
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-09-19
DOI:10.1016/j.bioadv.2025.214519
引用次数: 0
摘要
依西美坦(EXE)是一种不可逆的芳香酶抑制剂,是一种公认的治疗雌激素受体阳性(ER+)乳腺癌的药物。尽管已证实其临床益处,但其更广泛的治疗潜力受到诸如生物利用度差、血浆半衰期短和不良脱靶效应等因素的限制。纳米技术的最新进展提出了解决这些限制的有希望的策略。将EXE封装在纳米颗粒(NPs)中提供了一个新的平台,可以改善药物传递,增强肿瘤靶向性,并优化治疗效果。各种NP系统,包括脂基、聚合物和无机载体,已经被研究用于EXE的封装和输送。这些纳米制剂具有多种优点,如提高溶解度,持续和控制释放,选择性递送到恶性细胞。本文综述了体外和体内证据,支持负载exe的NPs具有优越的抗癌性能,以及它们在乳腺癌治疗中克服关键挑战的潜力。此外,本文还讨论了将EXE NPs整合到精准医学方法中的安全性、转化潜力和未来前景,为其与传统治疗方案的结合应用提供了见解,以改善乳腺癌的管理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
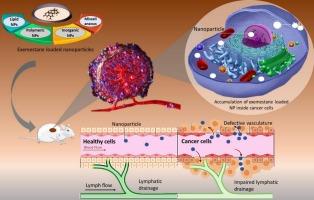
Exploring the efficacy and mechanisms of exemestane-loaded nanoparticles in targeted breast cancer therapy
Exemestane (EXE), an irreversible aromatase inhibitor, is a well-established therapeutic agent for the management of estrogen receptor-positive (ER+) breast cancer. Despite its proven clinical benefits, its broader therapeutic potential is limited by factors such as poor bioavailability, short plasma half-life, and undesirable off-target effects. Recent advances in nanotechnology have introduced promising strategies to address these limitations. Encapsulating EXE within nanoparticles (NPs) offers a novel platform to improve drug delivery, enhance tumor targeting, and optimize therapeutic efficacy. Various NP systems including lipid-based, polymeric, and inorganic carriers have been investigated for EXE encapsulation and delivery. These nanoformulations confer multiple advantages, such as improved solubility, sustained and controlled release, and selective delivery to malignant cells. The presented review examines the in vitro and in vivo evidence supporting the superior anticancer performance of EXE-loaded NPs, alongside their potential to overcome key challenges in breast cancer therapy. In addition, safety profiles, translational potential, and future perspectives for integrating EXE NPs into precision medicine approaches are discussed, offering insights into their application in combination with conventional treatment regimens for improved breast cancer management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


