毛毛象高氧化型倍半萜类二聚体:结构表征、DFT计算和抗肝癌潜能
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从毛毛象中分离出四种先前描述过的高度氧化的德曼烷型倍半萜类二聚体。通过综合光谱分析,结合实验电子圆二色性(ECD)和时变密度泛函理论(TDDFT)计算,明确了它们的平面结构和绝对构型。这些二聚体以o -醚键为特征,代表了首次报道的大象属衍生物的例子。在量子力学(QM)框架内进行Hartree-Fock能量计算,验证了结构的有效性。所有分离的倍半萜类二聚体对人肝癌细胞株HepG2和Hep3B均表现出中等抑制活性。其中化合物4的细胞毒性最强,IC50值分别为1.64 μM (HepG2)和4.85 μM (Hep3B)。此外,化合物4显著降低线粒体膜电位(MMP),表明其通过线粒体功能障碍诱导细胞凋亡。网络药理学和分子对接进一步表明,化合物4可能通过结合关键氨基酸残基与HSP90AA1相互作用,可能解释其药理活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
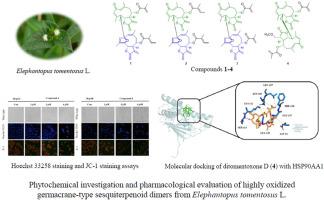
Highly oxidized germacrane-type sesquiterpenoid dimers from Elephantopus tomentosus L.: Structural characterization, DFT calculation and anti-hepatoma potential
Four previously undescribed highly oxidized germacrane-type sesquiterpenoid dimers were isolated from Elephantopus tomentosus. Their planar structures and absolute configurations were unequivocally elucidated through comprehensive spectroscopic analysis, combined with experimental electronic circular dichroism (ECD) and time-dependent density functional theory (TDDFT) calculations. These dimers, characterized by an O-ether linkage, represent the first reported examples of such derivatives from the genus Elephantopus. The structural validity was verified by performing Hartree-Fock energy calculations within the quantum mechanical (QM) framework. All isolated sesquiterpenoid dimers exhibited moderate inhibitory activity against human hepatocellular carcinoma cell lines (HepG2 and Hep3B). Notably, compound 4 demonstrated the most significant cytotoxicity, with IC50 values of 1.64 μM (HepG2) and 4.85 μM (Hep3B). Furthermore, compound 4 markedly reduced mitochondrial membrane potential (MMP), indicating its role in inducing apoptosis via mitochondrial dysfunction. Network pharmacology and molecular docking further indicated that compound 4 could interact with HSP90AA1 by binding to key amino acid residues, potentially explaining its pharmacological activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


