新型α-蒎烯类桉叶酚类化合物的合成及细胞毒活性研究
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
单萜烯及其衍生物通常被认为是广泛使用的廉价原料,可以转化为用于开发新药的有价值的化学品。在本研究中,以(+)-和(−)-α-蒎烯衍生的单萜类化合物和对卤素取代的芳香醛为起始原料,合成了一系列以甲硝基[3,2-c]吡喃和甲硝基[4,3-b]吡喃为支架的化合物。这些化合物含有1,4-和1,8-桉树脑-单萜烯的片段,具有广泛的生物效应。以8-乙酰氧基-6-羟甲基柠檬烯为原料,在105℃无溶剂干燥条件下,在K10的存在下合成了含有1,4-桉树脑片段的Methanofuro[3,2- C]pyrans 18。优化了含1,8-桉叶酚的甲吡喃[4,3-b]吡喃19的合成条件。结果表明,当K10(200℃干燥)在二氯甲烷中催化时,8-羟基-6-羟甲基柠檬烯与醛的反应对产物19有很高的选择性。研究了桉叶酚类化合物对HeLa、MCF7和A-172细胞的细胞毒活性。结果表明,化合物18b-d和19b-d的细胞毒活性取决于它们的(+)/(−)构型和特定结构中卤素原子的存在。活性最高的化合物-含Cl和Br取代基的甲烷吡喃[4,3-b]吡喃(+)-19c和(+)-19d对癌细胞具有高毒性,但对Vero细胞毒性很小。化合物(+)-19c和(+)-19d在MCF7和A-172细胞中具有细胞抑制作用或诱导凋亡细胞死亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
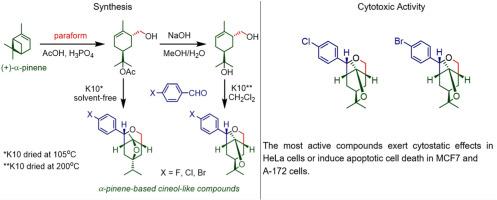
Synthesis and cytotoxic activity of new α-pinene-based cineol-like compounds
Monoterpenes and their derivatives are often considered as widespread and cheap raw materials for the transformation into the valuable chemicals using for the development of new drugs. In this study, a series of compounds with methanofuro[3,2-c]pyran and methanopyrano[4,3-b]pyran scaffolds were synthesized starting from (+)- and (−)-α-pinene-derived monoterpenoids and p-halogen substituted aromatic aldehydes. These compounds contain a fragment of 1,4- and 1,8-cineoles ‒ monoterpenes exhibited a wide range of biological effects. Methanofuro[3,2-c]pyrans 18 with a fragment of 1,4-cineole were synthesized from 8-acetoxy-6-hydroxymethyllimonene in the presence of K10 dried at 105 °C in solvent-free conditions. Optimized conditions for the synthesis of the methanopyrano[4,3-b]pyrans 19 bearing the 1,8-cineol moiety were developed. It was shown that the 8-hydroxy-6-hydroxymethyllimonene reactions with aldehydes proceed with high selectivity towards product 19 when catalyzed by K10 (dried at 200 °C) in methylene chloride. Cytotoxic activity of cineol-like compounds was studied against HeLa, MCF7 and A-172 cells. It was shown that cytotoxic activity of compounds 18b-d and 19b-d is depended on both their (+)/(−) configuration and halogen atom presence in a particular structure. The most active compounds - methanopyrano[4,3-b]pyrans (+)-19c and (+)-19d with Cl and Br substituents exhibited high toxicity to cancerous cells, but were little toxic to Vero cells. Compounds (+)-19c and (+)-19d can exert cytostatic effects in HeLa cells or induce apoptotic cell death in MCF7 and A-172 cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


