磷酸催化对醌与乙腈的区域选择性酰胺化
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了在温和条件下,磷酸催化乙腈酰胺化对醌类化合物(p-QMs)的高效、原子经济的方法。水作为氢原子和氧原子的唯一来源,在酰胺键形成中实现了100%的原子经济性。该反应在广泛的电子和空间多样性的p-QMs中表现出优异的通用性,促进了高效和高区域选择性的合成有合成价值的n -二芳基甲基取代的乙酰胺,收率很高。逐步对照实验和竞争性氘标记KIE研究阐明了反应途径,并基于累积的实验证据得出了一个合理的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
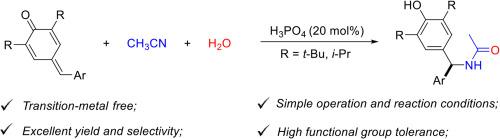
Phosphoric acid-catalyzed regioselective amidation of para-quinone methides with acetonitrile
An efficient and atom-economical phosphoric acid-catalyzed amidation of para-quinone methides (p-QMs) with acetonitrile has been developed under mild conditions. Water serves as the sole source of both hydrogen and oxygen atoms, enabling 100 % atom economy in amide bond formation. The reaction exhibits excellent generality across a broad spectrum of electronically and sterically diverse p-QMs, facilitating efficient and highly regioselective synthesis of synthetically valuable N-diarylmethyl-substituted acetamides in good to excellent yields. Step-by-step control experiments and competitive deuterium-labeling KIE studies elucidated the reaction pathway and led to a plausible mechanism based on cumulative experimental evidences.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


