ce掺杂MIL-101(Fe)衍生CoOx/MnOx@Fe2O3催化剂光热耦合催化降解丙酮和NO
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
与传统的热催化氧化技术相比,光热催化降解丙酮和转化一氧化氮(NO)的技术不仅降低了能耗,而且提高了降解效率,有效地克服了单一光催化或热催化技术的局限性。本文旨在通过优化锰钴(Mn-Co)的摩尔比和掺杂Ce对催化剂进行改性,控制催化剂的晶格氧活性和氧空位浓度,从而提高催化剂降解丙酮和NO的光催化和热催化性能。在240℃条件下,当Mn-Co摩尔比为4:2时,CoOx/MnOx@Fe2O3-2催化剂对丙酮和NO均表现出良好的催化活性,丙酮和NO的转化率分别为52%和63.8%。在优化Mn-Co摩尔比的基础上,采用共沉淀法将Ce掺杂到CoOx/MnOx@Fe2O3-2样品中,合成不同Ce掺杂量的样品。与其他样品相比,CeO2/CoOx/MnOx@Fe2O3-2-0.25 (nMn-Co:nCe = 1:0.25)样品的催化性能最高,在240℃下丙酮和NO的转化率分别达到60%和70%。此外,光热协同作用的内在机制基于Mars-van Krevelen氧化还原循环理论。本文章由计算机程序翻译,如有差异,请以英文原文为准。
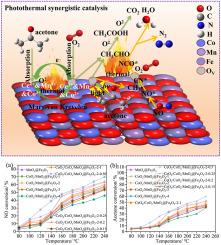
Ce-doped MIL-101(Fe)-derived CoOx/MnOx@Fe2O3 catalysts for photothermal coupled catalytic degradation of acetone and NO
The technology of photothermal catalytic degradation of acetone and conversion of nitrogen monoxide (NO) not only reduces energy consumption compared to traditional thermal catalytic oxidation technology but also improves degradation efficiency, effectively overcoming the limitations of single photocatalytic or thermal catalytic technology. This paper aims to control the lattice oxygen activity and oxygen vacancy concentration of the catalyst by optimizing the manganese-cobalt (Mn–Co) molar ratio and modifying the catalyst with Ce doping, thereby enhancing its photocatalytic and thermal catalytic performance for the degradation of acetone and NO. At 240 °C, when the Mn–Co molar ratio is 4:2, the CoOx/MnOx@Fe2O3-2 catalyst exhibits good catalytic activity for both acetone and NO, with conversion rates of 52% and 63.8% for acetone and NO, respectively. Based on the optimization of the Mn–Co molar ratio, Ce was doped into the CoOx/MnOx@Fe2O3-2 sample using the co-precipitation method to synthesize samples with different Ce doping amounts. The sample of CeO2/CoOx/MnOx@Fe2O3-2-0.25 (nMn-Co:nCe = 1:0.25) shows the highest catalytic performance compared with the other samples, with the conversion of acetone and NO reaching 60% and 70%, respectively, at 240 °C. Additionally, the intrinsic mechanism under photothermal synergy is based on the Mars-van Krevelen redox cycle theory.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


