受挫Lewis对非共轭末端二乙炔分子内碳-碳键形成和碳-硼环化的机理研究
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
受挫Lewis对(FLPs)与非共轭末端二乙炔表现出高度可变的反应性,导致1,2-抗加成产物,也导致分子内C-C偶联,随后随着Lewis碱基的变化产生C-B环化。然而,这些反应的详细机理尚未得到研究。本研究报告了一个详细的机制调查C-C偶联随后的1,6-庚二炔的C-B环化的FLPs。密度泛函理论计算揭示了三种不同的机制途径,包括1,1-碳硼化,以及1,2-碳硼化途径的两种替代变化。1,1-碳硼化反应涉及到相邻碳的c -c偶联[途径(a)],而1,2-碳硼化反应涉及到相邻碳的c -c偶联[途径(b)和(c)]。计算结果表明,1,1-碳硼化反应在能量上是有利的,区域选择性8元产物的形成受反应动力学效应的支配。不同的路易斯碱(PPh2[C6H3(CF3)2])和连接剂[di(丙炔)醚]的作用表明,通过1,1-碳硼化途径容易形成8元两性离子杂环产物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
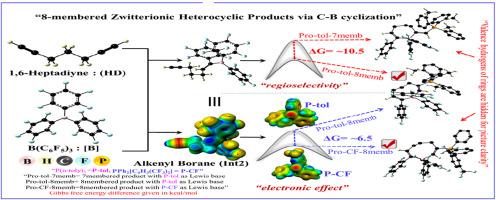
Mechanistic insights into intramolecular carbon–carbon bond formation and carbon–boron cyclization of non-conjugated terminal diacetylenes by frustrated Lewis Pairs: A DFT study
Frustrated Lewis pairs (FLPs) exhibit highly variable reactivity with non-conjugated terminal diacetylenes, leading to 1,2-anti-addition products and also to intramolecular C-C coupling followed by C-B cyclization with the variation in the Lewis bases. However, the detailed mechanisms of such reactions have not been examined. This study reports a detailed mechanistic investigation of C-C coupling followed by C-B cyclization of 1,6-heptadiyne by FLPs. Density functional theory calculations unveil three different mechanistic pathways, including 1,1-carboboration, and two alternative variations of 1,2-carboboration pathways. The 1,1-carboboration involves C-C coupling on geminal carbon [pathway (a)], while 1,2-carboboration involves vicinal C-C coupling [pathways (b) and (c)]. The computed results reveal that the 1,1-carboboration is energetically favoured and the formation of regioselective 8-membered product is governed by the kinetic effect in the reaction. The role of different Lewis bases (PPh2[C6H3(CF3)2) and linkers [di(propargyl)ether] suggests the ease to form the 8-membered zwitterionic heterocyclic products via 1,1-carboboration pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


