通过链走和CH活化,用乙酰苯胺催化远端分支和对映选择性内烯烃氢化
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了铱催化乙酰苯胺与内链烯烃的支链和对映选择性烷基化反应。本报告通过使用新开发的基于taddol的缺电子手性配体,解决了选择性CC键形成的关键挑战,并在产率和对构象比(高达91:9 er)之间实现了最佳平衡。与手性配体相比,使用1.5等量的[Ir(cod)2]NTf2可显著提高收率,并具有良好的区域选择性和对映体选择性。底物范围表明该方法广泛适用于各种乙酰苯胺和烯烃衍生物,包括功能化芳烃和含芳基或脂肪取代基的烯烃。本文章由计算机程序翻译,如有差异,请以英文原文为准。
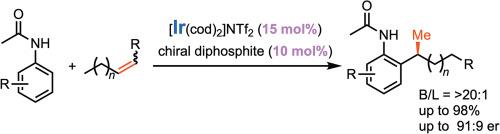
Ir-catalyzed distal branch- and enantioselective hydroarylation of internal alkenes using acetanilides via chain-walking and CH activation
This study presents the iridium-catalyzed branch- and enantioselective C![]() H alkylation of acetanilides with internal alkenes via chain-walking. This report addresses a key challenge in the selective C
H alkylation of acetanilides with internal alkenes via chain-walking. This report addresses a key challenge in the selective C![]() C bond formation by using a newly developed electron-deficient TADDOL-based chiral ligand and we achieved an optimal balance between yield and enantiomeric ratio (up to 91:9 er). The use of 1.5 equivalent amounts of [Ir(cod)2]NTf2 relative to chiral ligands significantly improved the yields along with perfect regioselectivity and good enantioselectivity. The substrate scope demonstrated the broad applicability of the method across various acetanilide and alkene derivatives, including functionalized arenes and alkenes bearing aryl or aliphatic substituents.
C bond formation by using a newly developed electron-deficient TADDOL-based chiral ligand and we achieved an optimal balance between yield and enantiomeric ratio (up to 91:9 er). The use of 1.5 equivalent amounts of [Ir(cod)2]NTf2 relative to chiral ligands significantly improved the yields along with perfect regioselectivity and good enantioselectivity. The substrate scope demonstrated the broad applicability of the method across various acetanilide and alkene derivatives, including functionalized arenes and alkenes bearing aryl or aliphatic substituents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


