Cu+优化FeHCF协同环境,增强高压储能能力
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
普鲁士蓝类似物(PBAs)因其成本低、理论比容量高而被认为是钠离子电池极好的正极材料之一,特别是富钠铁基PBAs (FeHCF)能提供更高的能量密度。FeHCF在高压下充放电平台稳定性较差(FeC6部分),这主要受其配位环境的影响。本研究采用接近Fe2+离子半径的Cu+(六配位)取代FeHCF,减少了fe6空位,优化了配位环境。低Cu+取代FeHCF (Cu+0.625)在8.5 mA/g时电化学性能最佳,可逆比容量为142 mA h/g, FeC6部分贡献大于68 mA h/g,优于未改性FeHCF和其他Cu2+取代FeHCF。现场试验证明了Cu+0.625的可逆结构稳定性,支持了其高压平台容量的稳定性。这种Cu+取代策略进一步丰富了富钠FeHCF配位环境优化的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
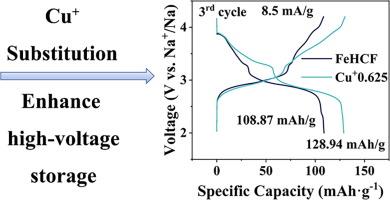
Cu+ optimizes the FeHCF coordination environment to enhance high-voltage energy storage
Prussian blue analogs (PBAs) are considered one of the excellent cathode materials for sodium-ion batteries due to their low cost and high theoretical specific capacity, especially sodium-rich iron-based PBAs (FeHCF) can provide higher energy density. FeHCF has a poor charge/discharge platform stability at high voltages (FeC6 moiety), which is mainly affected by its coordination environment. In this research, Cu+ (six-coordinated), which is close to the ionic radius of Fe2+, was used for substitution, the FeC6 vacancies of FeHCF were reduced, and the coordination environment was optimized. The low Cu+-substituted FeHCF (Cu+0.625) has an optimal electrochemical performance at 8.5 mA/g with a reversible specific capacity of 142 mA h/g and FeC6 moiety contribution of more than 68 mA h/g, which is superior to that of unmodified and other Cu2+-substituted FeHCFs. In situ tests demonstrate the reversible structural stability of the Cu+0.625, supporting the stability of their high-voltage platform capacity. This Cu+ substitution strategy further enriches the approach to optimize the coordination environment of sodium-rich FeHCF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


