聚合物参与的Li+溶剂化的多维优化使长循环锂金属电池稳定的聚合物塑料晶体电解质
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
丁二腈(SN)基聚合物塑料晶体电解质(PPCEs)被认为是锂金属电池的有前途的候选材料,但与锂金属存在严重的副反应。为此,我们提出了一种多维优化策略,以减轻SN与Li金属之间的副反应,并开发出一种高稳定性的聚乙烯碳酸酯基PPCE (PPCE- vec)。此外,我们还通过三种典型的聚酯类确定了聚合物多维结构对电解质稳定性的内在影响因素。通过x射线光电子能谱(XPS)分析,证实了原位聚合构建的PPCE- vec比基于聚乙烯醇的PPCE (PPCE- vca)和基于聚三氟丙烯酸乙酯的PPCE (PPCE- tfa)具有更好的稳定性。经过1400次循环后,PPCE-VCA-和ppce - tfa -使能的LiNi0.6Co0.2Mn0.2O2全电池的容量保持率分别为73.7%和46.9%。光谱分析和理论计算表明,PPCE-VEC羰基位点的高溶剂化能力、聚合物链的柔性以及电解质相的均质性有利于PPCE-VEC与Li+的最大竞争配位,从而减弱Li+ -SN的结合,形成富阴离子的溶剂化结构。优化后的聚合物Li+溶剂化增强了SN的稳定性,促进了富B/F固-电解质界面(SEI)的形成,从而显著提高了PPCE的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
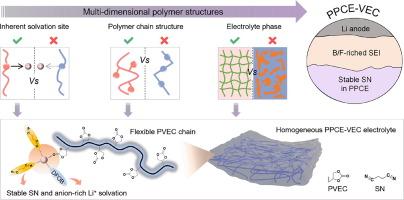
Multi-dimensional optimization of polymer-involved Li+ solvation enabling stable polymer plastic crystal electrolyte for long-cycle lithium metal batteries
Succinonitrile (SN)-based polymer plastic crystal electrolytes (PPCEs) are regarded as promising candidates for lithium metal batteries but suffer from serious side reactions with Li metal. Herein, we propose a multi-dimensional optimization strategy to alleviate the side reactions between SN and Li metal, and develop a highly stable poly-vinylethylene carbonate-based PPCE (PPCE-VEC). Moreover, we identify the intrinsic factors of multi-dimensional polymer structures on the electrolyte stability by three typical classes of polyesters. The PPCE-VEC constructed by in situ polymerization exhibits much better stability than poly-vinylene carbonate-based PPCE (PPCE-VCA) and poly-trifluoroethyl acrylate-based PPCE (PPCE-TFA), which is verified by its fewer SN-decomposition species in X-ray photoelectron spectroscopy (XPS) and outstanding full cell performance. The PPCE-VEC-enabled LiNi0.6Co0.2Mn0.2O2 full cell achieve 73.7 % capacity retention after 1400 cycles, which outperforms PPCE-VCA- and PPCE-TFA-enabled full cells (61.9 % and 46.9 %). Spectral analysis and theoretical calculation reveal that the high solvation ability of the carbonyl site, flexible polymer chain, and homogeneous electrolyte phase of PPCE-VEC are favorable to maximizing competition coordination with Li+ to weaken the Li+–SN binding and shape an anion-rich solvation structure. This optimized polymer-involved Li+ solvation enhances SN stability and facilitates the formation of B/F enriched solid-electrolyte interphase (SEI), thus significantly improving PPCE stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


