ALKBH5-IGF2BP2轴通过m6a稳定的CLSPN RNA介导前列腺癌进展和多西他赛耐药
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
前列腺癌(PCa)经常发展为去势抵抗性前列腺癌(CRPC),其中多西他赛(DTX)耐药性是一个主要挑战。我们研究了m6A去甲基化酶ALKBH5在这种耐药性中的作用。在CRPC临床样本中,ALKBH5的表达显著降低。功能上,ALKBH5过表达抑制了PCa细胞的增殖和迁移,而敲低ALKBH5则增强了这些作用,并增加了对DTX的抗性。相反,恢复ALKBH5或敲低m6A阅读器IGF2BP2的反向电阻。多组学分析发现,DNA复制应激调节因子CLSPN是一个关键的下游靶点。机制上,alkbh5介导的m6A去甲基化以依赖igf2bp2的方式降低CLSPN mRNA的稳定性。因此,低ALKBH5通过IGF2BP2稳定CLSPN,促进抗性。这些发现在临床样本和类器官模型中得到验证,表明ALKBH5-IGF2BP2轴通过m6a依赖性CLSPN调控转移性CRPC的DTX耐药性。靶向这一途径是克服DTX耐药的一种有希望的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
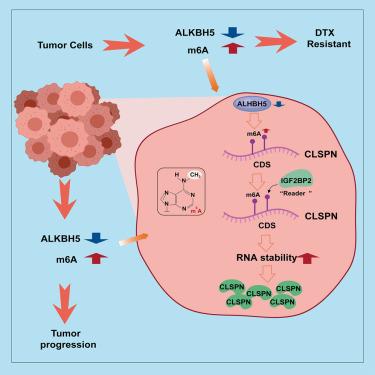
ALKBH5-IGF2BP2 axis mediates prostate cancer progression and docetaxel resistance via m6A-stabilized CLSPN RNA
Prostate cancer (PCa) often progresses to castration-resistant PCa (CRPC), where docetaxel (DTX) resistance is a major challenge. We investigated the role of the m6A demethylase ALKBH5 in this resistance. ALKBH5 expression was significantly reduced in CRPC clinical samples. Functionally, overexpressing ALKBH5 inhibited PCa cell proliferation and migration, while its knockdown enhanced these effects and increased DTX resistance. Conversely, restoring ALKBH5 or knocking down the m6A reader IGF2BP2 reversed resistance. Multi-omics analysis identified CLSPN, a DNA replication stress regulator, as a key downstream target. Mechanistically, ALKBH5-mediated m6A demethylation reduces CLSPN mRNA stability in an IGF2BP2-dependent manner. Low ALKBH5, therefore, stabilizes CLSPN via IGF2BP2, promoting resistance. These findings, validated in clinical samples and organoid models, demonstrate that the ALKBH5-IGF2BP2 axis modulates DTX resistance in metastatic CRPC through m6A-dependent regulation of CLSPN. Targeting this pathway represents a promising therapeutic strategy to overcome DTX resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


