揭示二氧化碳光还原BiVO4-Zn串联的高活性起源
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
在此,我们证明了基于bivo4的光阳极与金属Zn阴极的集成可以实现高性能的CO2还原,在模拟阳光(AM 1.5 G, 100 mW)下,CO的产率达到了创纪录的113.32 μmol cm−2 h−1,FECO达到了90.57%,并具有良好的稳定性。更重要的是,通过原位x射线光电子能谱(IS-XPS)和傅里叶变换红外反射(IS-FTIR)的结合,首次实现了对空间电荷分离/转移和H2O氧化和CO2还原的动态表面催化的直接观察。在光照射下,BiVO4光阳极上产生了电子-空穴对,空穴通过形成*OH和*OOH中间体迅速转移到光阳极表面参与析氧反应(OER)。同时,质子耦合电子转移到Zn阴极表面,通过形成*COOH和*CO中间体,驱动吸附的CO2分子还原成CO。因此,这项工作为对二氧化碳还原过程的基本理解提供了新的见解,这有助于未来高效碳固定系统的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
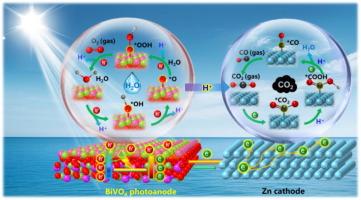
Unveiling the high-activity origins of BiVO4-Zn tandems for CO2 photoreduction
Herein, we demonstrated the integration of BiVO4-based photoanode with metallic Zn cathode for high-performance CO2 reduction, and a record CO production rate of 113.32 μmol cm−2 h−1 with a FECO of 90.57 % has been achieved under simulated sunlight (AM 1.5 G, 100 mW), accompanying with an excellent stability. More importantly, the direct observation of spatial charge separation/transfer and dynamic surface catalysis for both H2O oxidation and CO2 reduction has been firstly achieved by the combination of in situ X-ray photoelectron spectroscopy (IS-XPS) with Fourier transform infrared reflection (IS-FTIR). Under light irradiation, the electron-hole pairs have been generated on BiVO4 photoanode, and holes rapidly transfer to photoanode surfaces for participating in oxygen evolution reaction (OER) through the formation of *OH and *OOH intermediates. Simultaneously, the proton-coupled electron transfer to the Zn cathode surfaces drive the reduction of adsorbed CO2 molecules into CO via the formation *COOH and *CO intermediates. Thereby, this work offers new insights into fundamental understanding of CO2 reduction process, which facilitates the future development of highly efficient carbon fixation systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


