四面体Ni在NiCo2O4中电催化葡萄糖氧化生成八面体Co的自旋态工程
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
电催化葡萄糖氧化制高价值化学品为生物质增值提供了一条可持续的途径。镍基催化剂由于Ni和Co催化中心的固有活性而成为葡萄糖氧化反应的有希望的候选催化剂。然而,这些中心之间的动态演化和原子尺度的协同作用仍然难以捉摸。本文制备了泡沫镍支撑的NiCo2O4纳米片,其中Ni优先占据四面体位置来调节八面体Co的电子构型。实验和理论结果表明,四面体Ni的加入诱导了八面体Co的低至中自旋跃迁,从而优化了eg的轨道占用并稳定了活性位点。这种自旋态工程建立了Ni-Co协同催化中心,用于葡萄糖选择性氧化生成甲酸(FA)。在较高电位下(≥1.4 V vs. RHE),八面体Co重构为过多的CoOOH和CoO2活性物质,导致葡萄糖过度氧化为CO2,竞争析氧加剧。相比之下,在较低的电位下(<1.4 V vs. RHE),四面体Ni促进了电子在Ni - o - Co晶格上的离域,从而稳定了八面体Co对葡萄糖的吸附和氧化。建立了耦合电催化体系,在NiCo2O4阳极条件下,FA产率为80.7%,FE为91.3%;在Pt阴极条件下,FE为99.9%,H2析出率为696 μmol h−1,在1.35 V条件下,RHE反应2 h。这项工作对催化中心的自旋态调控提供了深入的认识,为合理设计催化剂提供了有价值的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
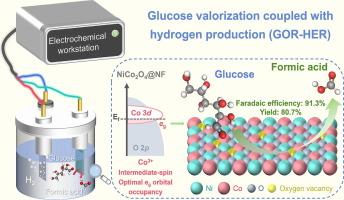
Spin-state engineering of octahedral Co via tetrahedral Ni in NiCo2O4 for electrocatalytic glucose oxidation to formate
Electrocatalytic glucose oxidation to high-value chemicals provides a sustainable route for biomass valorization. NiCo-based catalysts have emerged as promising candidates for glucose oxidation reaction owing to the intrinsic activity of Ni and Co catalytic centers. However, the dynamic evolution and atomic-scale synergy between these centers remain elusive. Herein, we fabricated NiCo2O4 nanosheets supported on nickel foam, where Ni preferentially occupies tetrahedral sites to regulate the electronic configuration of octahedral Co. Experimental and theoretical results demonstrate that the incorporation of tetrahedral Ni induces low-to-intermediate spin transition in octahedral Co, thereby optimizing eg orbital occupancy and stabilizing active sites. This spin-state engineering establishes Ni-Co synergistic catalytic centers for the selective oxidation of glucose to formate (FA). At higher potential (≥1.4 V vs. RHE), octahedral Co undergoes reconstruction into excessive active CoOOH and CoO2 species, resulting in glucose overoxidation to CO2 and intensified competitive oxygen evolution. In contrast, at lower potentials (<1.4 V vs. RHE), tetrahedral Ni facilitates electron delocalization across the Ni–O–Co lattice, thereby stabilizing octahedral Co for glucose adsorption and oxidation. Subsequently, a coupled electrocatalytic system was constructed, achieving 80.7 % FA yield with 91.3 % Faradaic efficiency (FE) at NiCo2O4 anode and H2 evolution rate of 696 μmol h−1 with 99.9 % FE at Pt cathode for 2 h under 1.35 V vs. RHE. This work provides a deep insight into spin-state regulation of the catalytic center, offering valuable guidance for rational catalyst design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


