CuBi2O4/Cu2O纳米稳定光电阴极在低偏压电位和太阳照射下促进CO2到甲基乙酸的转化
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
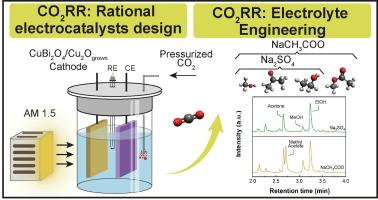
cu20基光电阴极的使用已经证明了这些地球上丰富的材料在光电化学CO2还原反应(CO2RR)中具有良好的活性,特别是在生产甲醇方面。然而,它们在长期器件中的应用受到严重光腐蚀的阻碍。为了解决这一限制,光电阴极设计结合肖特基势垒,异质结,和脚手架层已经探索。在这项工作中,CuBi2O4/CuO薄层被用作支撑Cu2O膜的支架,用于增强光电化学CO2RR。在co2饱和电解液中进行的光电化学测试表明,在0.55 V下,可逆氢电极(RHE)产生甲醇(CH3OH)的活性和稳定性最高,优于其他负电位。此外,本研究还强调了电解质工程可用于促进醋酸甲酯(CH3COOCH3)等替代产品的生成。CuBi2O4/CuO支架的存在对于实现这一途径至关重要,它既增强了稳定性,又改善了Cu2O表面的电荷转移。CH3COOCH3的生成归因于当溶液中存在乙酸时,局部修饰的微环境促进了酯化反应。这些发现突出了支架工程在改善光电阴极性能和电解质调节方面的作用,这些作用使产品选择性转向很少被探索的附加值化合物,如醋酸甲酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CuBi2O4/Cu2O nano-enabled stable photocathodes promoting CO2 to methyl acetate conversion under low bias potential and solar irradiation
The use of Cu2O-based photocathodes has demonstrated the promising activity of these earth-abundant materials for the photoelectrochemical CO2 reduction reaction (CO2RR), particularly in producing methanol. However, their application in long-term devices is hindered by severe photocorrosion. To address this limitation, photocathode designs incorporating Schottky barriers, heterojunctions, and scaffolding layers have been explored. In this work, a CuBi2O4/CuO thin layer was employed as a scaffold to support Cu2O films with either seeded or grown morphologies for enhanced photoelectrochemical CO2RR. Photoelectrochemical testing in CO2-saturated electrolyte revealed that 0.55 V vs. reversible hydrogen electrode (RHE) yielded the highest activity and stability for methanol (CH3OH) production, outperforming more negative potentials. Furthermore, the present work highlighted that electrolyte engineering can be used to promote the generation of alternative products such as methyl acetate (CH3COOCH3). The presence of CuBi2O4/CuO scaffold was critical for allowing this pathway, providing both enhanced stability and improved charge transfer on the Cu2O surface. The generation of CH3COOCH3 is attributed to locally modified microenvironments that facilitate the esterification reaction when acetate is present in solution. These findings highlight the role of scaffold engineering in improving photocathode performance and electrolyte tuning in steering product selectivity toward scarcely explored, added-value compounds such as methyl acetate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


