HNRNPA1促进TRIM37 mRNA稳定性,介导TRAF6泛素化,缓解骨关节炎
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
骨关节炎(OA)以进行性软骨退变为特征,涉及炎症和细胞死亡失调。rna结合蛋白HNRNPA1通过trim37介导的TRAF6泛素化在OA发病机制中的功能作用尚不清楚。方法采用内侧半月板失稳(DMM)诱导的OA小鼠模型,我们评估了软骨组织病理学、炎症和HNRNPA1、TRIM37和TRAF6的表达。分析白细胞介素-1β (IL-1β)处理的软骨细胞的活力、凋亡、焦亡和细胞外基质(ECM)合成。机制研究包括RNA荧光原位杂交(RNA- fish)、RNA免疫沉淀(RIP)、mRNA稳定性测定和TRIM37敲除后的共免疫沉淀(Co-IP),以检测TRAF6泛素化。结果soa进展与HNRNPA1、TRIM37表达降低、TRAF6表达升高相关。HNRNPA1过表达可减轻软骨降解、炎症和自噬损伤。在机制上,HNRNPA1稳定了TRIM37 mRNA,从而增强了TRIM37介导的TRAF6泛素化。这个级联抑制焦亡,同时激活自噬,赋予软骨保护。结论HNRNPA1/TRIM37/TRAF6轴调节自噬和焦亡之间的平衡,为缓解OA提供了新的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
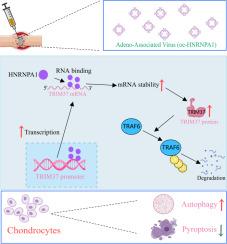
HNRNPA1 promotes TRIM37 mRNA stability and mediates TRAF6 ubiquitination to alleviate osteoarthritis
Background
Osteoarthritis (OA), characterized by progressive cartilage degeneration, involves inflammation and dysregulated cell death. The functional role of the RNA-binding protein HNRNPA1 via TRIM37-mediated TRAF6 ubiquitination in OA pathogenesis remains unclear.
Methods
Using a destabilization of the medial meniscus (DMM)-induced OA mouse model, we assessed cartilage histopathology, inflammation, and expression of HNRNPA1, TRIM37, and TRAF6. Interleukin-1β (IL-1β)-treated chondrocytes were analyzed for viability, apoptosis, pyroptosis, and extracellular matrix (ECM) synthesis. Mechanistic studies included RNA-fluorescence in situ hybridization (RNA-FISH), RNA immunoprecipitation (RIP), mRNA stability assays, and co-immunoprecipitation (Co-IP) following TRIM37 knockdown to examine TRAF6 ubiquitination.
Results
OA progression correlated with decreased HNRNPA1 and TRIM37 but increased TRAF6 expression. HNRNPA1 overexpression mitigated cartilage degradation, inflammation, and autophagy impairment. Mechanistically, HNRNPA1 stabilized TRIM37 mRNA, thereby enhancing TRIM37-mediated TRAF6 ubiquitination. This cascade suppressed pyroptosis while activating autophagy, conferring chondroprotection.
Conclusion
The HNRNPA1/TRIM37/TRAF6 axis regulates the balance between autophagy and pyroptosis, offering a novel therapeutic target to alleviate OA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


