超声介导的纳米颗粒和抗氧化剂促进卡介苗-谷氨酰胺生物膜的协同消除和组织修复
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
结核分枝杆菌(MTB)的生物膜形成通过阻碍药物渗透和逃避宿主免疫来增强抗生素耐药性。这对传统药物治疗提出了重大挑战,强调迫切需要新的治疗策略来克服结核分枝杆菌的生物膜介导的耐药性。本研究介绍了用于抗菌声动力治疗(aSDT)的低强度超声介导的左氧氟沙星(LEV)和负载过氧化氢酶(CAT)的PEG-PLGA纳米颗粒(LEV@CAT-NPs)的发展,通过利用BCG作为MTB的模型,提供了一种对抗BCG生物膜感染的创新策略。在抗感染治疗的后期补充n -乙酰半胱氨酸(NAC),促进巨噬细胞向M2型转化,促进组织修复。超声介导的LEV@CAT-NPs以及随后添加的NAC不仅增强了感染部位的修复,而且还导致组织中炎症反应的逐步消退。治疗方案诱导巨噬细胞极化向M2表型转移,调节细胞因子表达,减少促炎细胞因子,增加抗炎细胞因子,有助于恢复感染组织的氧化还原平衡。本研究提出了一种新的治疗策略,不仅针对耐药MTB,而且还促进组织修复,突出了其在感染管理中的双重作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
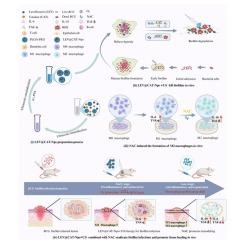
Synergistic elimination of bacillus Calmette-Guérin biofilm and tissue restoration facilitated by ultrasound-mediated nanoparticles and antioxidants
Biofilm formation in Mycobacterium tuberculosis (MTB) enhances antibiotic resistance by impeding drug penetration and evading host immunity. This poses a significant challenge to conventional drug therapies, highlighting the urgent need for novel treatment strategies to overcome MTB's biofilm-mediated resistance. This study introduces the development of low-intensity ultrasound-mediated levofloxacin (LEV) and catalase (CAT) -loaded PEG-PLGA nanoparticles (LEV@CAT-NPs) for antimicrobial sonodynamic therapy (aSDT), offering an innovative strategy to combat BCG biofilm infection, by utilizing BCG as a model for MTB. N-acetylcysteine (NAC) was supplemented during the latter stages of the treatment process of anti-infection therapy to facilitate the transformation of macrophages to the M2 phenotype and to promote tissue repair. Ultrasound-mediated LEV@CAT-NPs, along with the subsequent addition of NAC not only enhanced repair at the infection site but also led to a progressive resolution of the inflammatory response in tissues. The treatment regimen induced a shift in macrophage polarization towards the M2 phenotype and modulated cytokine expression, decreasing pro-inflammatory while increasing anti-inflammatory cytokines, which contributed to the restoration of redox balance in the infected tissues. This study proposes a novel therapeutic strategy that not only targets drug-resistant MTB but also promotes tissue repair, highlighting its dual role in infection management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


