含硫钒酸镁复合材料作为锌离子电池正极的两种氧化还原反应
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
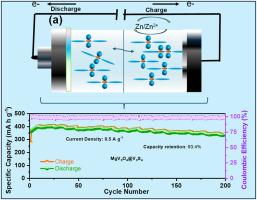
钒基材料因其优异的安全性被认为是锌离子电池的潜在阴极材料。虽然v基阴极的层状结构是不稳定的,但它会导致剧烈的容量衰减。v基材料的含硫是保护层结构和促进氧化还原反应的有效策略。本文采用微波辅助溶剂热法,设计了钒酸镁(MgV2O4)的硫掺杂,形成了钒酸镁和硫化钒(V3S4)的复合材料(即MgV2O4@V3S4),并将其用作水性ZIBs的阴极。为了进行比较,使用相同的合成程序,MgV2O4@V2O3样品也没有任何硫掺入。MgV2O4@V3S4阴极复合材料由于硫的正价转化而表现出优异的电化学性能。令人印象深刻的是,MgV2O4@V3S4阴极复合材料在500 mA g−1下,在200次循环中获得了340 mA h g−1的高放电容量(容量保持率:93.4%)。Zn//MgV2O4@V3S4电池在733 W kg - 1时的能量密度为442 Wh kg - 1,在132.6 Wh kg - 1时的能量密度为2690 W kg - 1,显示出良好的功率特性。离地XPS研究表明,MgV2O4@V3S4中锌离子的储存机制是通过V4+/V5+和S2−/S4+氧化还原反应实现的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Two species redox reaction in sulfur-incorporated magnesium vanadate composite as a cathode for aqueous zinc-ion batteries
The vanadium (V)- based materials are treated as potential cathodes for aqueous zinc-ion batteries (ZIBs) owing to their excellent safety feature. Although the layer structure of the V-based cathodes is unstable, it results in drastic capacity decay. Sulfur incorporation of V-based materials is an effective strategy to protect the layer structure as well as boost the redox reaction. Herein, we designed a sulfur-incorporation of magnesium vanadate (MgV2O4) to form the magnesium vanadate and vanadium sulfide (V3S4) composite (i.e., MgV2O4@V3S4) using a microwave-assisted solvothermal method and utilized it as a cathode for aqueous ZIBs. For the comparison, using the same synthesis procedures, the MgV2O4@V2O3 sample also prepared without any sulfur incorporation. The MgV2O4@V3S4 cathode composite demonstrates excellent electrochemical performance due to the positive valence conversion of sulfur. Impressively, the MgV2O4@V3S4 cathode composite obtains a high discharge capacity of 340 mA h g−1 over 200 cycles at 500 mA g−1 (capacity retention: 93.4 %). The Zn//MgV2O4@V3S4 cell exhibits a very impressive energy density of 442 Wh kg−1 at 733 W kg−1, and 2690 W kg−1 at 132.6 Wh kg−1, revealing the good power features. Ex-situ XPS study reveals that the zinc-ion storage mechanism in MgV2O4@V3S4 occurs through V4+/V5+ and S2−/S4+ redox reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


