致癌突变驱动的代谢-免疫调节轴:甲状腺癌精准治疗的潜在前景
IF 9.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Reviews on cancer
Pub Date : 2025-09-24
DOI:10.1016/j.bbcan.2025.189459
引用次数: 0
摘要
癌基因促进癌症的发展,其特定的激活突变说明了启动和介导甲状腺癌(TC)进展的机制。研究主要集中在癌基因如何通过影响下游信号通路促进不同TC亚型的发展。靶向治疗效果显著;然而,它们通常通过反馈激活或代偿信号旁路诱导耐药。最近的证据表明,甲状腺癌基因启动和介导TC进展,并通过诱导代谢重编程和免疫微环境重塑,促进不同TC亚型的耐药。因此,我们提出了“癌基因-代谢-免疫轴”的概念。我们讨论了癌基因驱动的代谢重编程和肿瘤免疫微环境重塑(TIME)及其相互作用诱导TC进展、耐药和免疫逃避的分子机制。最后,我们系统地评估和总结了针对关键癌基因、代谢催化剂、免疫检查点(ic)和联合治疗的潜在策略,以提高靶向治疗TC的疗效并克服耐药性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
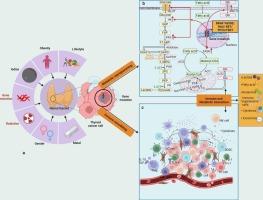
Oncogenic mutation-driven metabolism-immunity regulatory axis: Potential prospects for thyroid cancer precision therapy
Oncogenes enhance cancer development, and their specific activating mutations exemplify the mechanisms that initiate and mediate thyroid cancer (TC) progression. Research has predominantly focused on how oncogenes promote the development of different TC subtypes by influencing the downstream signaling pathways. Targeted therapies show significant efficacy; however, they often induce drug resistance through feedback activation or compensatory signaling bypasses. Recent evidence indicates that thyroid oncogenes initiate and mediate TC progression, and contribute to drug resistance in distinct TC subtypes through induced metabolic reprogramming and immune microenvironment remodeling. Hence, we propose the concept “Oncogene-Metabolism-Immunity axis.” We discussed the molecular mechanisms by which oncogene-driven metabolic reprogramming and tumor immune microenvironment Remodeling (TIME), and their mutual interactions, induce TC progression, drug resistance, and immune evasion. Finally, we systematically evaluated and summarized potential strategies targeting key oncogenes, metabolic catalysts, immune checkpoints (ICs), and combination therapies to enhance the efficacy of targeted treatments for TC and overcome drug resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Reviews on cancer
医学-生化与分子生物学
CiteScore
17.20
自引率
0.00%
发文量
138
审稿时长
33 days
期刊介绍:
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer encompasses the entirety of cancer biology and biochemistry, emphasizing oncogenes and tumor suppressor genes, growth-related cell cycle control signaling, carcinogenesis mechanisms, cell transformation, immunologic control mechanisms, genetics of human (mammalian) cancer, control of cell proliferation, genetic and molecular control of organismic development, rational anti-tumor drug design. It publishes mini-reviews and full reviews.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


