mn3o4增强双功能水凝胶用于轻度温控肿瘤消融和成骨
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
由于肿瘤复发和成骨不良,肿瘤切除后骨重建仍然具有挑战性。虽然光热支架可以消融肿瘤(通过高热)和刺激骨形成(通过低热),但很难平衡这两种作用——过热会损伤组织,而热量不足则不能消除肿瘤。在这里,我们开发了一种可注射的Mn3O4增强水凝胶(GM/OD/Mn3O4)来解决这个问题。通过生物矿化技术首次合成的Mn3O4纳米片具有较高的光热效率和模拟酶活性(葡萄糖氧化酶/过氧化物酶样)。该水凝胶能够适应不规则缺陷,并在酸性肿瘤微环境中释放Mn3O4。通过催化级联,它消耗ATP并抑制热休克蛋白,克服肿瘤的耐热性,并在温和温度(43°C)下实现有效消融。较低的近红外功率(40°C)进一步促进成骨。在体内,水凝胶在抑制肿瘤复发的同时促进骨再生,为术后骨修复提供了双重功能策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
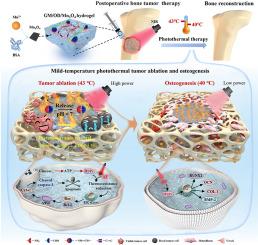
Mn3O4-potentiated bifunctional hydrogel for mild temperature-controlled tumor ablation and osteogenesis
Postoperative bone reconstruction after tumor resection remains challenging due to tumor recurrence and poor osteogenesis. Although photothermal scaffolds can ablate tumors (via high heat) and stimulate bone formation (via mild heat), balancing these effects is difficult—excessive heat damages tissue, while insufficient heat fails to eliminate tumors. Here, we develop an injectable Mn3O4-enhanced hydrogel (GM/OD/Mn3O4) to address this. Mn3O4 nanosheets, firstly synthesized via biomineralization, exhibit high photothermal efficiency and enzyme-mimetic activity (glucose oxidase/peroxidase-like). The hydrogel adapts to irregular defects and releases Mn3O4 in acidic tumor microenvironments. Through catalytic cascades, it depletes ATP and inhibits heat shock proteins, overcoming tumor thermoresistance and enabling effective ablation at mild temperatures (43 °C). Lower NIR power (40 °C) further enhances osteogenesis. In vivo, the hydrogel suppresses tumor recurrence while promoting bone regeneration, offering a dual-functional strategy for postoperative bone repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


