反应性纳米医学策略实现胰腺癌精确治疗
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
胰腺导管腺癌(PDAC)是胰腺癌的主要亚型,是世界上最致命的恶性肿瘤之一,其5年生存率低于13%。其不良预后源于复杂的解剖屏障、致密且异质性的肿瘤微环境(TME)以及复杂的分子调控网络,这些因素共同阻碍了早期发现并限制了治疗效果。纳米医学通过增强药物负荷、改善递送、抵消耐药性和实现刺激反应性控制提供了有希望的解决方案。值得注意的是,刺激反应性纳米疗法已经成为一种变革性的策略,通过内源性TME信号(如酸性pH值、氧化还原梯度、缺氧、酶过表达)或外源性触发(如超声波、光、磁场)的激活来实现精确的药物释放。内源性反应系统在肿瘤部位自主激活,增强肿瘤内药物积累,减少脱靶效应,而外源性反应平台通过外部调节实现时空控制。多反应系统整合了这两种机制,以实现动态和协同的治疗效果,对PDAC治疗具有重要的前景。本文综述了刺激反应纳米治疗PDAC的最新进展,详细介绍了它们的激活机制、生物医学应用以及内源性、外源性和多反应模式的治疗潜力。它进一步讨论了将这些技术转化为临床实践的当前挑战和未来方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
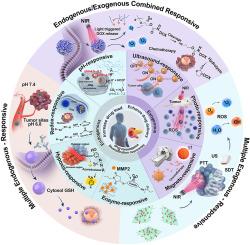
Responsive nanomedicine strategies achieve pancreatic cancer precise theranostics
Pancreatic ductal adenocarcinoma (PDAC), the predominant subtype of pancreatic cancer, ranks among the deadliest malignancies worldwide, with a 5-year survival rate remaining below 13 %. Its poor prognosis stems from complex anatomical barriers, a dense and heterogeneous tumor microenvironment (TME), and intricate molecular regulatory networks that collectively hinder early detection and limit therapeutic efficacy. Nanomedicine offers promising solutions by enhancing drug loading, improving delivery, counteracting drug resistance, and enabling stimuli-responsive control. Notably, stimuli-responsive nanotherapeutics have emerged as a transformative strategy, achieving precise drug release through activation by endogenous TME cues (e.g., acidic pH, redox gradients, hypoxia, enzyme overexpression) or exogenous triggers (e.g., ultrasound, light, magnetic fields). Endogenous-responsive systems autonomously activate at tumor sites, enhancing intratumoral drug accumulation and reducing off-target effects, while exogenous-responsive platforms enable spatiotemporal control through external modulation. Multi-responsive systems integrate both mechanisms to achieve dynamic and synergistic therapeutic effects, holding significant promise for PDAC theranostics. This review summarizes recent advances in stimuli-responsive nanotherapeutics for PDAC, detailing their activation mechanisms, biomedical applications, and theranostic potential across endogenous, exogenous, and multi-responsive modalities. It further discusses current challenges and future directions for translating these technologies into clinical practice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


