利用骨髓源性抑制细胞的双重免疫调节功能重塑骨关节炎治疗的炎症微环境
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
骨关节炎(OA)的发病机制受到免疫动力学失调的深刻影响,其中持续的白介素-17 (IL-17)/T辅助17 (Th17)细胞介导的炎症与失败的再生过程协调,使关节永久破坏。在这里,我们揭示了髓源性抑制细胞(MDSCs)作为双期调节因子的作用,在OA进展中矛盾地协调炎症升级和组织修复。骨性关节炎小鼠关节内给药MDSCs可扩增IL-17依赖性炎症级联反应和趋化因子驱动的白细胞募集,揭示出环境依赖性促炎表型。出乎意料的是,MDSC缺失未能减轻关节损伤,这意味着它们在OA发病机制中不可或缺且多方面的作用。从机制上讲,MDSCs通过上调精氨酸酶-1来使M2巨噬细胞极化,在其炎症活性的同时培养再生生态位,从而表现出功能可塑性。为了解决这一问题,我们开发了一种生物响应的水凝胶-微球系统,该系统集成了转化生长因子β1 (TGF-β1)和白细胞介素-1 β1抗体(抗il -1β)负载的介孔二氧化硅纳米颗粒(MSNs)。这个时空控制的平台选择性地抑制mdsc介导的Th17细胞扩增,同时利用其内在能力驱动M2巨噬细胞极化和软骨形成。由此产生的从促炎到促再生微环境的转变显著减轻了OA模型中的软骨侵蚀并恢复了关节完整性。我们的研究结果重新定义了MDSCs作为OA中的双功能免疫协调者,并建立了精确的生物材料引导免疫解码作为一种范式转换的治疗策略。通过拮抗细胞因子的传递来设计MDSCs的可塑性,这项工作为退行性关节疾病的微环境重塑提供了蓝图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
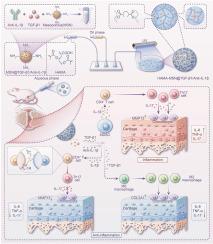
Harnessing the dual immunomodulatory function of myeloid-derived suppressor cells to reshape the inflammatory microenvironment for osteoarthritis therapy
Osteoarthritis (OA) pathogenesis is profoundly influenced by dysregulated immune dynamics, where persistent interleukin-17 (IL-17)/T helper 17 (Th17) cell mediated inflammation coordinates with failed regenerative processes to perpetuate joint destruction. Here, we unveil the role of myeloid-derived suppressor cells (MDSCs) as dual-phase regulators that paradoxically orchestrate both inflammatory escalation and tissue repair in OA progression. Intra-articular administration of MDSCs in OA mice amplified IL-17 dependent inflammatory cascades and chemokine-driven leukocyte recruitment, revealing a context-dependent pro-inflammatory phenotype. Unexpectedly, MDSC depletion failed to attenuate joint damage, implying their indispensable yet multifaceted role in OA pathogenesis. Mechanistically, MDSCs exhibited functional plasticity by upregulating arginase-1 to polarize M2 macrophages, fostering a regenerative niche alongside their inflammatory activity. To resolve this duality, we developed a bio-responsive hydrogel-microsphere system integrating transforming growth factor β1 (TGF-β1) and interleukin-1 β1 antibody (anti–IL-1β) loaded mesoporous silica nanoparticles (MSNs). This spatiotemporally controlled platform selectively suppressed MDSC-mediated Th17 cell expansion while harnessing their intrinsic capacity to drive M2 macrophage polarization and chondrogenesis. The resultant shift from a pro-inflammatory to pro-regenerative microenvironment significantly attenuated cartilage erosion and restored joint integrity in OA models. Our findings redefine MDSCs as bifunctional immune orchestrators in OA and establish precision biomaterial guided immune decoding as a paradigm-shifting therapeutic strategy. By engineering MDSCs plasticity through antagonistic cytokine delivery, this work provides a blueprint for microenvironment remodeling in degenerative joint diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


