多功能纳米平台作为铁下垂纳米诱导剂用于转移性前列腺癌的靶向识别和成像引导治疗
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
转移性前列腺癌的精确检测和有效治疗仍是临床面临的重大挑战。铁下垂为转移性前列腺癌的治疗带来了有希望的治疗策略,有效诱导前列腺癌细胞铁下垂是提高治疗效果的关键。在此,我们开发了一种多功能纳米平台Fe/Au纳米点-bombesin (FGN-BBN)作为铁下垂纳米诱导剂,通过“开源节流”策略产生大量ROS诱导铁下垂,用于转移性PCa的靶向成像引导治疗。一方面,FGN-BBN作为一种高效的仿生纳米酶和光热剂,表现出良好的pod样活性,通过光热增强化学动力学治疗(CDT)产生丰富的活性氧(ROS)诱导铁凋亡,达到“开源”的目的。另一方面,FGN-BBN表现出gpx样活性,消耗肿瘤微环境中过表达的谷胱甘肽(GSH),从而阻止ROS的中和,达到“抑制”作用。此外,bombesin促进纳米酶靶向传递到转移性PCa细胞,协同增强铁下垂活性。在诊断方面,FGN-BBN具有靶向识别能力,可实现多模式生物成像,包括荧光(FL)、计算机断层扫描(CT)和磁共振成像(MRI),可实现肿瘤定位的“可视化”和实时成像引导治疗。综上所述,多功能纳米平台整合了多酶活性、靶向识别、多模式成像、光热治疗和CDT来诱导高效的铁凋亡,为转移性前列腺癌提供了有效的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
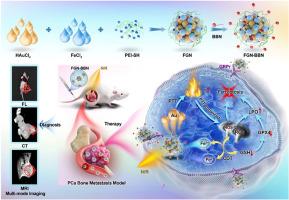
Multifunctional nanoplatform as nano-inducer of ferroptosis for targeted recognition and imaging-guided therapy of metastatic prostate cancer
Metastasis prostate cancer (PCa) precision detection and effective treatment remain significant challenge in clinic. Ferroptosis brought promising therapeutic strategy for the treatment of metastatic PCa, effectively inducing ferroptosis in PCa cells represents key to improve therapeutic efficacy. Herein, we developed a multifunctional nanoplatform Fe/Au nanodots-bombesin (FGN-BBN) as the ferroptosis nano-inducer to generate large amount of ROS to induce ferroptosis through an “open-source throttling” strategy for targeted imaging-guided therapy of metastatic PCa. On the one hand, FGN-BBN serves as an efficient biomimetic nanozyme and photothermal agent, exhibiting great POD-like activity and generating abundant reactive oxygen species (ROS) via photothermal-enhanced chemodynamic therapy (CDT) to induce ferroptosis, which is achieving “open source” aspect. On the other hand, FGN-BBN exhibit GPx-like activity that depletes overexpressed glutathione (GSH) within the tumor microenvironment, thereby preventing the neutralization of ROS and achieving the “throttling” effect. Furthermore, bombesin facilitates targeted delivery of the nanozyme to metastatic PCa cells, synergistically enhancing ferroptosis activity. In terms of diagnosis, FGN-BBN possesses targeted recognition capabilities and enables multimode bioimaging including fluorescence (FL), computed tomography (CT), and magnetic resonance imaging (MRI), allowing for the “visualization” of tumor localization and real-time imaging-guided therapy. In summary, the multifunctional nanoplatform integrates multienzyme activity, targeted recognition, multimodal imaging, photothermal therapy, and CDT to induce high-efficiency ferroptosis, offering an effective theranostic strategy for metastatic PCa.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


