一种新的叶酸受体靶向姜黄素纳米递送系统:精确抑制MRCKβ抑制卵巢癌增殖和免疫逃逸的双重治疗策略
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
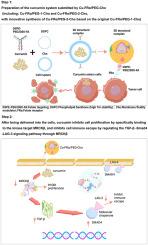
姜黄素是从姜黄根茎中提取的一种天然多酚类化合物,具有抗炎、抗氧化、抗肿瘤、抗菌、抗病毒和神经保护等多种生物活性。但口服吸收差、生物利用度低,严重限制了其临床应用。为了解决这些挑战,本研究合成了一种新的叶酸靶向姜黄素负载材料cu - fr α/PEG-2-CHO。该传递系统通过叶酸受体(FRα)介导的活性靶向(FRα表示叶酸受体α亚型)显著提高肿瘤细胞摄取效率。此外,聚乙二醇(PEG)改性和醛(-CHO)功能化进一步提高了其水溶性和稳定性。实验结果表明,该材料不仅能显著提高姜黄素的吸收率和生物利用度,还能通过靶向递送机制增强姜黄素的抗肿瘤活性。本研究开发了一种新型叶酸受体靶向姜黄素纳米递送系统(CU-FRα/PEG-2-CHO),并系统比较了两种姜黄素纳米材料(CU-FRα/PEG-1-CHO和CU-FRα/PEG-2-CHO)的抗肿瘤作用,结果表明CU-FRα/PEG-2-CHO可显著抑制A2780和HO8910PM卵巢癌细胞株的生长。CU-FRα/PEG-2-CHO靶向肿瘤细胞后,释放姜黄素,机制显示其特异性靶向cdc42结合激酶β (MRCKβ, IC50 = 2.12 μM),并通过表面等离子体共振(SPR)、等温滴定量热法(ITC)和细胞热移测定(CESTA)证实了其结合特性。分子对接分析表明,MRCKβ与姜黄素的atp结合袋精确结合。进一步的转录组学分析表明,MRCKβ沉默显著影响TGF-β-SMAD4-LAG-3信号通路,CU-FRα/PEG-2-CHO通过调节MRCKβ- apc - smad4 - ctla -4信号轴发挥其治疗作用,抑制肿瘤细胞增殖和免疫逃避。动物实验证实,每2天给药1 mg/kg的CU-FRα/PEG-2-CHO显著抑制肿瘤生长,且具有剂量依赖效应。值得注意的是,虽然顺铂(5 mg/kg / 2天)和αPD-L1 (5 mg/kg / 2天)也有一定的治疗效果,但其抑瘤效果明显低于CU-FRα/PEG-2-CHO。这些发现不仅阐明了一种新的叶酸受体靶向姜黄素纳米脂质体的作用机制,也为开发以mrck β为靶点的卵巢癌治疗策略提供了重要依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A novel folate receptor-targeted curcumin nano-delivery system: A dual therapeutic strategy for precise inhibition of MRCKβ to suppress ovarian cancer proliferation and immune escape
Curcumin is a natural polyphenolic compound extracted from the rhizomes of Curcuma longa, exhibiting diverse biological activities, including anti-inflammatory, antioxidant, antitumor, antibacterial, antiviral, and neuroprotective effects. However, its clinical application is severely limited by poor oral absorption and low bioavailability. To address these challenges, this study synthesized a novel folate-targeted curcumin-loaded material—CU-FRα/PEG-2-CHO. This delivery system significantly enhances tumor cell uptake efficiency through folate receptor (FRα)-mediated active targeting (FRα denotes the folate receptor alpha subtype). Additionally, polyethylene glycol (PEG) modification and aldehyde (-CHO) functionalization further improve its water solubility and stability. Experimental results demonstrate that this material not only substantially increases curcumin's absorption rate and bioavailability but also enhances its antitumor activity via targeted delivery mechanisms. This study developed a new type (significantly improving various performances) folate receptor-targeted curcumin nano-delivery system (CU-FRα/PEG-2-CHO) and systematically compared the antitumor effects of two curcumin nanomaterials (CU-FRα/PEG-1-CHO and CU-FRα/PEG-2-CHO), demonstrating that CU-FRα/PEG-2-CHO significantly inhibited the growth of A2780 and HO8910PM ovarian cancer cell lines. After targeting tumor cells, CU-FRα/PEG-2-CHO released curcumin, which was mechanistically shown to specifically target Cdc42-binding kinase β (MRCKβ, IC50 = 2.12 μM), with its binding characteristics confirmed by surface plasmon resonance (SPR), isothermal titration calorimetry (ITC), and cellular thermal shift assay (CESTA). Molecular docking analysis revealed that MRCKβ precisely binds to the ATP-binding pocket of curcumin. Further transcriptomic analysis demonstrated that MRCKβ silencing significantly affected the TGF-β-SMAD4-LAG-3 signaling pathway, and CU-FRα/PEG-2-CHO exerted its therapeutic effects by regulating the MRCKβ-APC-SMAD4-CTLA-4 signaling axis to suppress tumor cell proliferation and immune evasion. Animal experiments confirmed that CU-FRα/PEG-2-CHO administration at a dose of 1 mg/kg every two days significantly inhibited tumor growth with a dose-dependent effect. Notably, although cisplatin (5 mg/kg every two days) and αPD-L1 (5 mg/kg every two days) also showed certain therapeutic effects, their tumor suppression efficacy was significantly lower than that of CU-FRα/PEG-2-CHO. These findings not only elucidate the mechanism of a novel folate receptor-targeted curcumin nanoliposome but also provide important evidence for developing MRCKβ-targeted therapeutic strategies against ovarian cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


