一种用于内源性自我补氧的仿生纳米平台,促进靶向消融动脉粥样硬化斑块中活化巨噬细胞的声动力治疗
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
动脉粥样硬化(AS)是一种慢性炎症,发病率和死亡率增高。基于纳米材料的声动力疗法(SDT)作为一种无创治疗方法显示出相当大的潜力。由于细胞微环境中氧气可用性不足,SDT的功效往往受到活性氧(ROS)生成不稳定的限制。本研究通过在巨噬细胞膜(mmms)上包裹载氯e6 (Ce6)的中空二氧化锰(HMnO2)纳米颗粒,构建仿生纳米平台(HMnO2-Ce6@MM; HMC@MM),以提高活性氧水平,有效消融活化的巨噬细胞。HMC@MM被主动转运到动脉粥样硬化斑块中活化的巨噬细胞。在斑块微酸性且富含过氧化氢(H2O2)的环境中,HMnO2与H2O2反应生成Mn2+和氧气。Mn2+适用于磁共振成像检测不稳定斑块。在内源性自补氧条件下,HMC@MM-mediated SDT可产生足够的ROS,进而启动线粒体功能障碍,诱导活化的巨噬细胞凋亡。对活化的巨噬细胞进行消融术,不仅可以缓解炎症环境,而且凋亡片段还可以引发斑块内新鲜巨噬细胞的efferocysis,从而有效控制不稳定斑块。HMC@MM-mediated SDT策略在控制动脉粥样硬化斑块进展方面具有综合作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
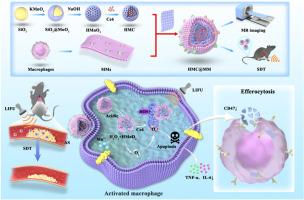
A biomimetic nanoplatform for endogenous self-replenishing of oxygen to promote sonodynamic therapy for targeted ablation of activated macrophages in atherosclerotic plaques
Atherosclerosis (AS) is a chronic inflammatory condition associated with increased morbidity and mortality. Nanomaterial-based sonodynamic therapy (SDT) shows considerable potential as a noninvasive treatment for AS. The efficacy of SDT is often limited by the unstable generation of reactive oxygen species (ROS) due to inadequate oxygen availability in the cellular microenvironment. In this study, a biomimetic nanoplatform (HMnO2-Ce6@MM; HMC@MM) was constructed by coating hollow manganese dioxide (HMnO2) nanoparticles loaded with Chlorine e6 (Ce6) with macrophage membranes (MMs) to increase the ROS level for effective ablation of activated macrophages. HMC@MM is actively transported to activated macrophages in atherosclerotic plaques. In the slightly acidic and rich hydrogen peroxide (H2O2) environment of plaques, HMnO2 reacts with H2O2 to produce Mn2+ and oxygen. Mn2+ is applicable in magnetic resonance imaging to detect unstable plaques. Under the condition of endogenous self-replenishing oxygen, HMC@MM-mediated SDT can produce sufficient ROS, subsequently initiating mitochondrial dysfunction and inducing the apoptosis of activated macrophages. Ablation of activated macrophages not only alleviated the inflammatory environment but the apoptotic fragments also triggered efferocytosis of fresh macrophages in the plaque, thereby effectively controlling unstable plaques. The HMC@MM-mediated SDT strategy has comprehensive effects on controlling atherosclerotic plaque progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


