高性能水锌离子电池阳极用锌铋合金表面氧化锌纳米管阵列的制备
IF 4.2
3区 工程技术
Q2 ELECTROCHEMISTRY
引用次数: 0
摘要
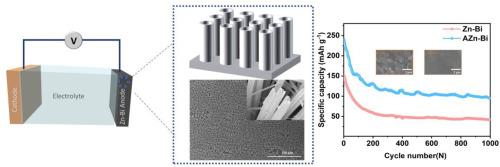
在这项工作中,采用阳极氧化技术在不同铋含量(0.5、1、1.5和2 wt%)的锌铋(Znbi)合金表面制备了氧化锌(ZnO)纳米管阵列。探讨了铋含量对ZnO纳米管阵列形貌的影响。通过优化阳极氧化参数,电解液中含有50mm碳酸氢钠和乙二醇,水与乙二醇的体积比为9:1。在锌表面合成了直径均匀(395.2±53.6 nm)的ZnO纳米管阵列。随着铋含量的增加,ZnO (Zn-0.5Bi)的平均纳米管直径从487.2±54.2 nm降低到293.4±26.5 nm。阳极氧化后的Znbi合金在水溶液锌离子电池中表现出良好的循环稳定性,在1 a g−1下循环1000次后,其容量保持在95.04 mAh g−1。阳极氧化的锌铋电极在对称电池中也表现出良好的循环稳定性,在1ma cm−2时过电位仅为28.5 mV。这项工作为设计高稳定性的锌基阳极提供了一种有前途的方案本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fabrication zinc oxide nanotubular arrays on the surface of zinc‑bismuth alloy for high-performance aqueous zinc-ion battery anodes
In this work, zinc oxide (ZnO) nanotubular arrays were fabricated on the surface of zinc‑bismuth (Zn![]() bi) alloys with different bismuth contents (0.5, 1, 1.5, and 2 wt%) using an anodization technique. The influence of bismuth content on the morphology of the ZnO nanotubular arrays was explored. By optimizing anodization parameters in an electrolyte containing 50 mM sodium bicarbonate, ethylene glycol, and volume ratio of water to ethylene glycol is 9:1. ZnO nanotubular arrays with uniform nanotubular diameters (395.2 ± 53.6 nm) were synthesized on the zinc surface. The increasing of content of bismuth reduced the average nanotubular diameter from 487.2 ± 54.2 nm for ZnO (Zn-0.5Bi) to 293.4 ± 26.5 nm for ZnO (Zn
bi) alloys with different bismuth contents (0.5, 1, 1.5, and 2 wt%) using an anodization technique. The influence of bismuth content on the morphology of the ZnO nanotubular arrays was explored. By optimizing anodization parameters in an electrolyte containing 50 mM sodium bicarbonate, ethylene glycol, and volume ratio of water to ethylene glycol is 9:1. ZnO nanotubular arrays with uniform nanotubular diameters (395.2 ± 53.6 nm) were synthesized on the zinc surface. The increasing of content of bismuth reduced the average nanotubular diameter from 487.2 ± 54.2 nm for ZnO (Zn-0.5Bi) to 293.4 ± 26.5 nm for ZnO (Zn![]() 2Bi). When used as an anode, the anodized Zn
2Bi). When used as an anode, the anodized Zn![]() bi alloy demonstrated good cycling stability in aqueous zinc-ion battery, maintaining a capacity of 95.04 mAh g−1 after 1000 cycles at 1 a g−1. The anodized Zn
bi alloy demonstrated good cycling stability in aqueous zinc-ion battery, maintaining a capacity of 95.04 mAh g−1 after 1000 cycles at 1 a g−1. The anodized Zn![]() bi electrode also exhibited excellent cycling stability in a symmetric cell, with an overpotential of only 28.5 mV at 1 mA cm−2. This work provides a promising protocol for designing highly stable zinc-based anodes
bi electrode also exhibited excellent cycling stability in a symmetric cell, with an overpotential of only 28.5 mV at 1 mA cm−2. This work provides a promising protocol for designing highly stable zinc-based anodes
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochemistry Communications
工程技术-电化学
CiteScore
8.50
自引率
3.70%
发文量
160
审稿时长
1.2 months
期刊介绍:
Electrochemistry Communications is an open access journal providing fast dissemination of short communications, full communications and mini reviews covering the whole field of electrochemistry which merit urgent publication. Short communications are limited to a maximum of 20,000 characters (including spaces) while full communications and mini reviews are limited to 25,000 characters (including spaces). Supplementary information is permitted for full communications and mini reviews but not for short communications. We aim to be the fastest journal in electrochemistry for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


