近亲繁殖的蝾螈基因组揭示了蝾螈类两栖动物行为、发育和再生的分子机制
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
蝾螈为研究脊椎动物的进化、发育和再生提供了极好的模型。为了进一步提高蝾螈在生物学上的模式生物地位,我们对一种近交蝾螈(Pleurodeles waltl)的20 Gb基因组进行了初步测序。作为本研究的一部分,由于大量插入重复序列,包括蝾螈特异性卫星DNA, Hoxd11-d13基因间区扩展了超过1 Mb。有趣的是,Myod和Bmp4这两种通常与脊椎动物发育有关的基因在蝾螈中是缺失的。编码性信息素配体的Sodefrin前体样因子基因的共同选择表明,蝾螈的生殖行为多样化。此外,Shh的肢体增强子MFCS1/ZRS即使位于距离启动子约5mb的位置,也能保持其功能。此外,我们还在该增强子中发现了一个与肢体再生相关的功能性顺式元件。蝾螈基因组对两栖动物的进化、行为、发育和再生提供了至关重要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
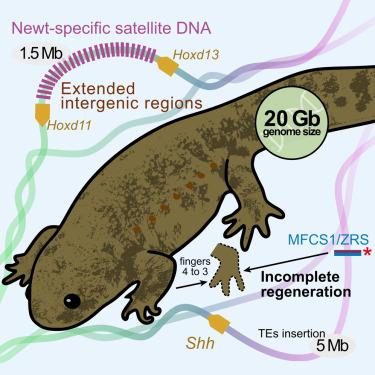
The inbred newt genome unveils molecular mechanisms of behavior, development, and regeneration in urodele amphibians
Salamanders provide excellent models for studying vertebrate evolution, development, and regeneration. To further advance the newt as a model organism in biology, we conducted draft genome sequencing of 20 Gb of an inbred newt (Pleurodeles waltl). As part of this study, the Hoxd11–d13 intergenic region is expanded by over 1 Mb owing to the massive insertion of repetitive sequences including newt-specific satellite DNA. Interestingly, Myod and Bmp4, genes that are typically involved in vertebrate development, are absent in salamanders. Co-option of Sodefrin Precursor-like Factor genes, which encode sex pheromone ligands, suggests a diversification of reproductive behavior among salamanders. Moreover, a limb enhancer of Shh, MFCS1/ZRS, retains its function, even though it is positioned approximately 5 Mb away from the promoter. Furthermore, we have identified a functional cis-element potentially associated with limb regeneration in this enhancer. The newt genome yields crucial insights into amphibian evolution, behavior, development, and regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


