基于l -甲基甘油内酯的高统计PLGAs: pH荧光传感器监测共聚物微观结构对水解降解的影响
IF 6.3
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
聚乳酸-羟基乙酸(PLGAs)在生物医学上的应用受到这些共聚物水解降解过程中酸化的严重阻碍,这可能导致术后并发症,如周围组织的毒性酸性反应。PLGAs通常由丙交酯(LA)和乙醇酸酯(GL)开环共聚(co-ROP)得到;LA和GL的反应性差异较大,形成的共聚物统计性较低。聚乳酸/乙醇酸酯(L/G)比、分子量特征和共聚物的微观结构对聚乳酸/乙醇酸酯的生物降解有很大影响。人们认为,长(G)n段的存在推动了PLGAs的快速水解,形成低分子量有机酸和相对高分子量(L)n残余物,这些都不利于PLGAs的生物相容性。在目前的工作中,我们提出在l-LA的co-ROP中使用l-甲基乙醇酸(l-MeGL)来随机化PLGAs。制备了l-LA/l-MeGL摩尔比为85:15 (PLMG 85/15)和70:30 (PLMG 70/30)的共聚物,以及l-MeGL均聚物PLMG 0/100;合成l-LA/GL共聚物PLGA 85/15和PLGA 70/30进行比较。根据1H和13C NMR数据,l- megl基PLGAs不含n >; 2的(G)n段,DSC研究显示共聚物具有较高的统计性。采用pH荧光传感器4-PYMPO,采用激光扫描显微镜对PLGA薄膜的水解降解进行了研究。这些研究揭示了L- megl - PLGAs和基于gl的PLGAs在水解行为上的显著差异:L- megl - PLGAs在L/G比相等的情况下被证明是非酸化聚酯,这将为其在生物医学上的应用开辟广阔的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
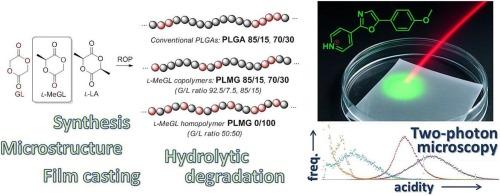
Highly statistical PLGAs based on L-methylglycolide: the impact of copolymer microstructure on hydrolytic degradation monitored by pH fluorescent sensor
Biomedical applications of poly(lactic-co-glycolic acid)s (PLGAs) are significantly hindered by acidification during hydrolytic degradation of these copolymers, which can cause postoperative complications such as toxic acidic response in surrounding tissues. PLGAs are usually obtained by ring-opening copolymerization (co-ROP) of lactide (LA) and glycolide (GL); marked difference in reactivity of LA and GL results in formation of copolymers with low statisticity. Biodegradation of PLGAs is strongly influenced by lactate/glycolate (L/G) ratios, molecular weight characteristics and microstructure of copolymers. It is believed that the presence of long (G)n segments is driving fast hydrolysis of PLGAs with a formation of low-MW organic acids and relatively high-MW (L)n remnants, none of that is good for biocompatibility of PLGAs. In the present work, we proposed the use of l-methylglycolide (l-MeGL) in co-ROP with l-LA to randomize PLGAs. Copolymers with l-LA/l-MeGL molar ratios of 85:15 (PLMG 85/15) and 70:30 (PLMG 70/30), as well as l-MeGL homopolymer PLMG 0/100, were prepared; l-LA/GL copolymers PLGA 85/15 and PLGA 70/30 were synthesized for a comparison. According to 1H and 13C NMR data, l-MeGL-based PLGAs did not contain (G)n segments with n > 2, DSC studies revealed high statisticity of copolymers. Investigations of hydrolytic degradation of thin PLGA films were conducted by laser scanning microscopy with the use of pH fluorescent sensor 4-PYMPO. These studies revealed the marked difference in hydrolytic behavior of l-MeGL- and GL-based PLGAs: with equal L/G ratios, l-MeGL-based PLGAs proved to be non-acidifying polyesters, which will open wide prospects for their biomedical use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


