结构和位点特异性n -聚糖改变定义了大鼠胸腺衰老的糖蛋白组学景观
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
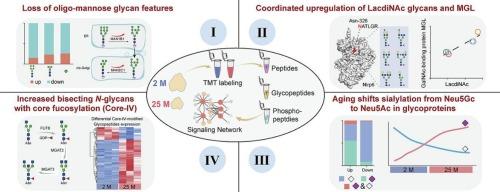
胸腺老化是免疫衰老和全身生理衰退的标志,但其潜在的分子机制仍不完全清楚。在这项研究中,我们通过整合定量全局和磷酸化蛋白质组数据集,对衰老大鼠胸腺进行了高分辨率的糖蛋白质组学分析。通过消除蛋白表达变化后鉴定出484个上调和866个下调的位点特异性n -聚糖,研究揭示了四种主要的年龄相关聚糖特征模式,包括低聚甘露糖聚糖显著减少,LacdiNAc聚糖显著增加,与galnac结合蛋白MGL升高密切相关;主要上调neu5ac修饰的糖肽,以及在不同免疫过程中出现在不同糖蛋白上的neu5gc修饰的糖肽,以及上调核心聚焦的分割n -聚糖(core- iv)。衰老相关的糖基化重塑主要由糖基化相关酶的改变驱动,并调节免疫和衰老相关的信号通路。总之,这些发现强调了糖基化在胸腺衰老中的功能重要性,并建立了糖基化动力学的高分辨率分子图谱,作为研究免疫衰退和年龄相关疾病的宝贵资源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural and site-specific N-glycan alterations define the glycoproteomic landscape of thymic aging in rats
Thymic aging is a hallmark of immune senescence and systemic physiological decline, yet its underlying molecular mechanisms remain incompletely understood. In this study, we performed a high-resolution glycoproteomic analysis of the aging rat thymus by integrating with quantitative global and phospho-proteome datasets. By identifying 484 upregulated and 866 downregulated site-specific N-glycans after eliminating their protein expression changes, the study revealed four major age-associated glycan feature patterns, including markedly reduced oligo-mannose glycans, significantly increased LacdiNAc glycans with a strong correlation with elevated GalNAc-binding protein MGL, predominantly upregulated Neu5Ac-modified glycopeptides along with largely downregulated Neu5Gc-modified glycopeptides occurring at distinct glycoproteins for different immune processes, as well as upregulated bisecting N-glycans with core-fucosylation (Core-IV). Aging-related glycosylation remodeling is largely driven by altered glycosylation-related enzymes, as well as modulates immune and aging-related signaling pathways. Together, these findings highlight the functional importance of glycosylation in thymic aging and establish a high-resolution molecular map of glycosylation dynamics as a valuable resource for investigating immune decline and age-related diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


