Shh的模式和增殖作用被划分在不同的外泌体上
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
摘要
Sonic hedgehog基因(Shh)在脊髓发育过程中是一个关键的形态因子,协调腹侧神经模式和祖细胞增殖。这些不同的结果是如何确定的仍然是难以捉摸的。在这里,我们发现Shh是通过两个生化和功能上不同的外泌体池分泌的。致密的囊泡片段sh - p150驱动Smoothened-Gli1信号以建立腹侧祖细胞身份,而较轻的囊泡片段sh - p450激活smoothened - g - αi依赖通路,增强祖细胞增殖而不诱导腹侧死亡。我们发现Rab7是一种晚期内体调节因子,对sh - p150的生物发生和脊索介导的腹侧神经模式至关重要。Rab7的缺失使分泌偏向增殖的sh - p450库,并破坏形态发生信号传导。这些发现确立了外泌体包装作为一个分子开关,在其有丝分裂和形态发生作用之间切换Shh。通过将外泌体生物发生与发育结果联系起来,我们的工作揭示了一种新的机制,该机制在神经管发育过程中保护模式形成和祖细胞扩张之间的平衡,这对发育障碍和Shh信号失调的疾病环境都有影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
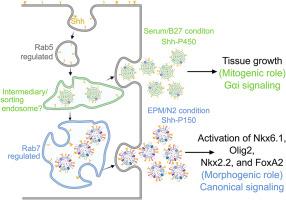
The patterning and proliferation roles of Shh are partitioned on distinct exosomes
Sonic hedgehog (Shh) is a pivotal morphogen in spinal cord development, orchestrating both ventral neural patterning and progenitor proliferation. How these distinct outcomes are specified has remained elusive. Here, we uncover that Shh is secreted via two biochemically and functionally distinct exosomal pools. A dense vesicle fraction, Shh-P150, drives Smoothened–Gli1 signalling to establish ventral progenitor identities, while a lighter pool, Shh-P450, activates a Smoothened–Gαi–dependent pathway that enhances progenitor proliferation without inducing ventral fate. We identify Rab7, a late endosomal regulator, as essential for Shh-P150 biogenesis and for notochord-mediated ventral neural patterning. Loss of Rab7 biases secretion toward the proliferative Shh-P450 pool and disrupts morphogenetic signalling. These findings establish exosomal packaging as a molecular switch that toggles Shh between its mitogenic and morphogenetic roles. By linking exosome biogenesis to developmental outcomes, our work reveals a novel mechanism that safeguards the balance between pattern formation and progenitor expansion during neural tube development, with implications for both developmental disorders and disease contexts where Shh signalling is misregulated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


