双靶向白蛋白纳米颗粒联合递送低剂量紫杉醇和PCSK9抑制剂治疗黑色素瘤
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
免疫抑制微环境和受损抗原呈递是黑色素瘤治疗的重大挑战。本研究探讨了通过同时诱导肿瘤细胞免疫原性细胞死亡(ICD)和增强抗原呈递来克服这些障碍的策略。我们开发了透明质酸/R8-RGD双修饰白蛋白纳米颗粒(HR/LDPP-NPs),旨在共同递送低剂量紫杉醇(PTX)和PCSK9抑制剂PF-06446846。PTX诱导ICD,释放钙调蛋白(CRT)、ATP和HMGB1危险信号,从而将肿瘤细胞转化为原位抗原源,并启动树突状细胞介导的t细胞应答,类似于固有的疫苗效应。同时,抑制PCSK9显著上调肿瘤MHC-I的表达,抵消免疫逃避。这种将原位疫苗接种与增强抗原呈递相结合的协同方法,显著增强了抗肿瘤免疫。体外和体内实验表明,这些时空协调的双靶向纳米颗粒在显著降低毒性的同时取得了可观的治疗效果,为黑色素瘤治疗提供了一种新的化学免疫治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
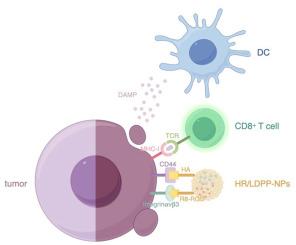
Dual-targeted albumin nanoparticles for the Co-delivery of low-dose paclitaxel and PCSK9 inhibitor in melanoma treatment
The immunosuppressive microenvironment and impaired antigen presentation represent significant challenges in melanoma treatment. This study explores strategies to overcome these barriers by simultaneously inducing tumor cell immunogenic cell death (ICD) and enhancing antigen presentation. We developed hyaluronic acid/R8-RGD dual-modified albumin nanoparticles (HR/LDPP-NPs) designed to co-deliver low-dose paclitaxel (PTX) and the PCSK9 inhibitor PF-06446846. PTX induces ICD, releasing calreticulin (CRT), ATP, and HMGB1 danger signals, thereby transforming tumor cells into an in situ antigen source and initiating dendritic cell-mediated T-cell responses, akin to an intrinsic vaccine effect. Simultaneously, the inhibition of PCSK9 significantly upregulates tumor MHC-I expression, counteracting immune evasion. This synergistic approach, combining in situ vaccination with enhanced antigen presentation, significantly amplifies antitumor immunity. Both in vitro and in vivo experiments demonstrate that these spatiotemporally coordinated dual-targeting nanoparticles achieve substantial therapeutic efficacy with significantly reduced toxicity, presenting a novel chemo-immunotherapeutic strategy for melanoma treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


