揭开LaVO4/Pd-gC3N4纳米复合材料的结构相变和电化学水分裂
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
开发经济高效的双功能电催化剂是实现电化学水分解制氢的关键。在这项研究中,我们报道了一种纳米棒状钒酸镧(LaVO4, LaV)与掺杂钯的石墨氮化碳(Pd-gCN)集成,形成一种杂化LaV/Pd-gCN复合催化剂。同步x射线衍射(SXRD)结果表明,LaV与Pd-gCN结合后结晶为一独居石型结构,并发生了微妙的结构转变,增强了其催化性能。钯与石墨氮化碳(g-C3N4)的掺入显著提高了其导电性,并引入了额外的活性位点,促进了电荷转移和反应动力学。电化学分析表明,LaV/Pd-gCN复合材料具有优异的双功能性能,在碱性介质中,在100 mA cm−2下,析氢反应(HER)和析氧反应(OER)的过电位分别为290 mV和410 mV。该复合材料还表现出优异的稳定性,在HER和OER连续运行100小时后仍保持85%以上的初始活性。性能的增强是由于La、V、Pd和g-C3N4基体之间的协同作用,促进了有利的电子结构和界面电荷转移。这些发现突出了LaV/Pd-gCN作为一种有前途的双功能电催化剂的潜力,为贵金属基系统提供了一种可行的替代方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
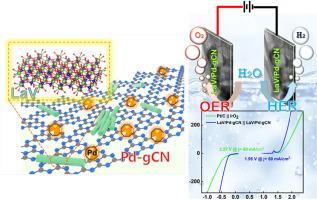
Unraveling the structural phase transition and electrochemical water splitting of LaVO4/Pd-gC3N4 nanocomposite
The development of cost-effective and efficient bifunctional electrocatalysts is vital for sustainable hydrogen production via electrochemical water splitting. In this study, we report the synthesis of a nanorod-shaped lanthanum vanadate (LaVO4, LaV) integrated with palladium-doped graphitic carbon nitride (Pd-gCN) to form a hybrid LaV/Pd-gCN composite catalyst. Synchrotron X-ray diffraction (SXRD) reveals that LaV crystallizes in a monazite-type structure and undergoes a subtle structural transformation when combined with Pd-gCN, enhancing its catalytic properties. The incorporation of Pd with graphitic carbon nitride (g-C3N4) significantly improves its electrical conductivity and introduces additional active sites, facilitating charge transfer and reaction kinetics. Electrochemical analysis demonstrates outstanding bifunctional performance of the LaV/Pd-gCN composite, with low overpotentials of 290 mV for the hydrogen evolution reaction (HER) and 410 mV for the oxygen evolution reaction (OER) at 100 mA cm−2 in alkaline media. The composite also exhibits excellent stability, retaining over 85 % of its initial activity after 100 h of continuous operation for both HER and OER. The enhanced performance is attributed to the synergistic interaction among La, V, Pd, and the g-C3N4 matrix, which promotes favorable electronic structures and interfacial charge transfer. These findings highlight the potential of LaV/Pd-gCN as a promising bifunctional electrocatalyst for overall water splitting, offering a viable alternative to noble metal-based systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


