人门静脉内皮细胞的分离和永生化:一种研究内脏血管系统的新研究工具
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
肝硬化深刻影响肝外血管系统,特别是改变门静脉系统,导致门静脉压力增加,门静脉侧枝和门静脉血栓形成,从而增加肝病的发病率和降低生存率。尽管肝硬化的肝内血管变化已经得到了很好的研究,但由于人门静脉的不可达性和临床前模型的不理想,对内脏区肝外改变的分子见解仍然有限。在这里,我们的目标是分离、表征和永生化原代人门静脉内皮细胞(pvec),以增强对肝脏疾病病理生理变化的理解,并为未来的药物测试建立平台。方法采用胰蛋白酶化、机械划伤和流式细胞术分离肝移植过程中获得的门静脉内皮细胞(spvecs, n = 12)和下腔静脉内皮细胞(ICV, n = 9)。通过基因和蛋白质标记表达的表征以及评估血管生成能力(管形成)、迁移能力(伤口愈合)和乙酰化低密度脂蛋白摄取的功能分析,证实了EC的身份。用表达SV40大t抗原的慢病毒颗粒永生化pvec (iPVECs)。结果分离的pvec证实了典型的内皮形态和功能,表达了标志蛋白和功能。pvec表现出与ICVEC和系统性ECs不同的转录组学特征,丰富了血管重构和应激反应的途径。在20多个传代中,iPVECs保留了内皮细胞的特性,并保留了ppec特异性的转录组特征。结论我们成功地分离、表征和永生化了pvec,为研究内脏血管疾病创造了一种新的工具。这些细胞保留了与全身静脉内皮细胞不同的转录组独特性,使研究肝脏疾病的血管功能障碍机制和支持转化研究成为可能。影响和意义体门高压和血管并发症是肝硬化发病率的主要驱动因素,但由于对人类门静脉组织的接触有限和模型不完善,对肝外血管机制仍知之甚少。通过分离、表征和永生化原代人门静脉内皮细胞,我们建立了第一个可再生的、与疾病相关的研究内脏血管生物学的平台。这些永生化门静脉内皮细胞保留了与全身静脉细胞不同的内皮特性和转录组特征,为肝脏疾病中的血管重塑和应激反应提供了独特的见解。这一资源使机制发现和药物测试旨在改善门静脉高压和相关并发症的结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
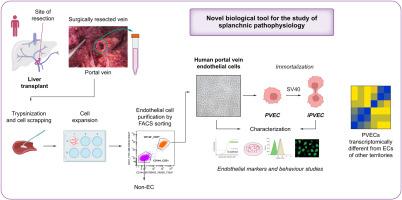
Isolation and immortalization of human portal vein endothelial cells: A novel research tool for studying splanchnic vasculature
Background & Aims
Cirrhosis profoundly impacts extrahepatic vasculature, particularly altering the portal venous system, leading to increased portal pressure, portosystemic collaterals, and portal vein thrombosis, which heightens morbidity and reduces survival in liver disease. Although intrahepatic vascular changes in cirrhosis are well studied, molecular insights into extrahepatic alterations in the splanchnic region remain limited owing to the inaccessibility of the human portal vein and suboptimal preclinical models. Here, we aim to isolate, characterize, and immortalize primary human portal vein endothelial cells (PVECs) to enhance understanding of pathophysiological changes during liver disease and establish a platform for future drug testing.
Methods
PVECs (n = 12) and inferior cava vein (ICV, n = 9) endothelial cells (ECs) were isolated from human portal vein or ICV, obtained during hepatic transplantation, using trypsinization, mechanical scratching, and FACS. EC identity was confirmed through characterization of gene and protein marker expression as well as functional assays assessing angiogenic capacity (tube formation), migratory ability (wound closure), and acetylated low-density lipoprotein uptake. PVECs were immortalized (iPVECs) with lentiviral particles expressing the SV40 large T-antigen.
Results
Isolated PVECs confirmed classical endothelial morphology and functionality, expressing hallmark proteins and functions. PVECs exhibited a distinct transcriptomic profile from ICVEC and systemic ECs, enriched in pathways for vascular remodeling and stress response. iPVECs retained endothelial identity and preserved the PVEC-specific transcriptomic traits across more than 20 passages.
Conclusions
We successfully isolated, characterized, and immortalized PVECs, creating a novel tool to study splanchnic vascular diseases. These cells retain transcriptomic uniqueness distinct from systemic venous ECs, enabling investigation of vascular dysfunction mechanisms in liver disease and supporting translational research.
Impact and implications
Portal hypertension and vascular complications are major drivers of morbidity in cirrhosis, yet extrahepatic vascular mechanisms remain poorly understood due to limited access to human portal vein tissue and inadequate models. By isolating, characterizing, and immortalizing primary human portal vein endothelial cells, we establish the first renewable, disease-relevant platform for studying splanchnic vascular biology. These immortalized portal vein endothelial cells preserve endothelial identity and transcriptomic signatures distinct from systemic venous cells, providing unique insights into vascular remodeling and stress responses in liver disease. This resource enables mechanistic discovery and drug testing aimed at improving outcomes in portal hypertension and related complications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


