基于浓度池的纳米颗粒表征方法的发展
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
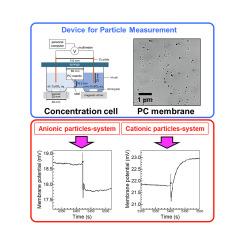
本研究介绍了一种利用浓度池的创新颗粒测量装置,该装置由两个半电池组成,由带有100 nm孔的轨迹蚀刻聚碳酸酯膜隔开,两个铜电极,不同浓度的CuSO4溶液以及与计算机接口的万用表。SiO2纳米颗粒胶体掺入低浓度半电池中,导致纳米颗粒堵塞孔隙,导致膜电位降低,这与SiO2颗粒浓度相关。由这种相关性推导出的颗粒浓度的检出限随着SiO2粒度的增大而减小。考虑到颗粒浓度可以按重量换算为胶体溶液中的颗粒含量,因此确定可以使用检测限下颗粒含量和粒径的校准曲线来估计颗粒大小。此外,引入带正电荷的SiO2颗粒增强了膜的表面电荷,导致膜电位在最初下降后逐渐增加,超过基线。这一观察结果提出了一种测定具有未知表面电荷的粒子的电荷极性的潜在方法。本研究证明了使用浓度池作为一种直接和经济有效的颗粒测量方法的可行性,具有确定颗粒大小和表面电荷的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development of a concentration cell-based method for nanoparticle characterization
This study introduces an innovative particle measurement device utilizing a concentration cell, which comprises two half-cells separated by a track-etched polycarbonate membrane with 100 nm pores, two copper electrodes, CuSO4 solutions of varying concentrations, and a multimeter interfaced with a computer. The incorporation of SiO2 nanoparticle colloids into the low-concentration half-cell led to pore obstruction by the nanoparticles, resulting in a reduction in the membrane potential, which correlated with the SiO2 particle concentration. The detection limit for the particle concentration, derived from this correlation, decreased as the SiO2 particle size increased. Given that the particle concentration can be converted to the particle content in the colloid solution by weight, it was determined that the particle size could be estimated using a calibration curve of the particle content and particle size at the detection limit. Additionally, the introduction of positively charged SiO2 particles enhanced the surface charge of the membrane, causing a gradual increase in the membrane potential beyond the baseline, following an initial decline. This observation suggests a potential method for determining the charge polarity of particles with unknown surface charges. This study demonstrates the viability of employing a concentration cell as a straightforward and cost-effective method for particle measurement, with the capability to ascertain the particle size and surface charge.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


