基于纳米扇形NiCoP的析氢和尿素氧化双功能电催化剂
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
尿素氧化反应(UOR)结合析氢反应(HER)取代传统的析氧反应(OER)是一种很有前途的低能制氢策略。在这项研究中,我们通过两步水热磷化方法开发了一种新型纳米片组装的扇形NiCoP电催化剂。得益于其独特的电子结构和丰富的活性位点,NiCoP催化剂只需要1.12 V(相对于RHE)的UOR和70 mV的过电位就可以达到10 mA·cm−2的电流密度。值得注意的是,即使在连续操作50 h后,活性也没有明显下降。此外,当在尿素辅助电解槽(HER||UOR)中使用时,催化剂在仅1.37 V的电池电压下达到10 mA·cm−2。这项工作为开发低成本、高能效的尿素电解制氢提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
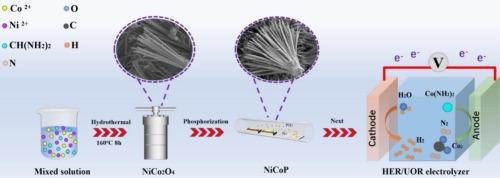
Bifunctional electrocatalysts based on nano-fan-shaped NiCoP for hydrogen evolution and urea oxidation reactions
Replacing the conventional oxygen evolution reaction (OER) with the urea oxidation reaction (UOR) coupled with hydrogen evolution reaction (HER) represents a highly promising strategy for low-energy hydrogen production. In this study, we developed a novel nanosheet-assembled fan-shaped NiCoP electrocatalyst via a two-step hydrothermal-phosphidation method. Benefiting from its unique electronic structure and abundant active sites, the NiCoP catalyst requires only 1.12 V (vs. RHE) for UOR and an overpotential of 70 mV for HER to reach a current density of 10 mA·cm−2. Remarkably, no obvious degradation in activity was observed even after 50 h of continuous operation. Moreover, when employed in a urea-assisted electrolyzer (HER||UOR), the catalyst achieves 10 mA·cm−2 at a cell voltage of merely 1.37 V. This work provides new insights into the development of low-cost and energy-efficient urea electrolysis for hydrogen production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


