大豆分离蛋白和豌豆分离蛋白复合材料的纤颤机理:重点研究结构和流变学性质
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
植物蛋白原纤维在食品工业中具有巨大的发展潜力,近年来引起了人们的广泛关注。然而,在原纤维形成过程中,外源植物蛋白之间的相互作用在很大程度上仍未被探索。在酸性加热条件下(pH 2.0, 85℃,0-24 h),将大豆分离蛋白和豌豆分离蛋白以不同的比例(1:2,1:1,2:1)进行pH转移处理,制备了新型复合原纤维(PSF)。SPI和PPI在PSF内表现出竞争生长机制,主导蛋白成分决定了纤颤过程。当加热12 h时,SPI与PPI比为1:1的PSF具有最强的纤颤能力和最佳的流变特性,纤维转化率超过60%,β-片含量为45.21%。微观结构分析表明,增加PPI比例可使大豆蛋白原纤维表面更光滑(Rq = 1.33 nm),有效抑制大豆蛋白原纤维的表面凝胶化倾向。FTIR分析证实,氢键和疏水相互作用是纤维形成的主要驱动力,PPI的加入增强了纤维网络的结构稳定性。分子对接验证了PPI与SPI之间的结合潜力,为后续的纤颤治疗奠定了理论基础。本研究创新性地设计了结构和功能可调的植物蛋白原纤维,以扩大其在食品工业中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
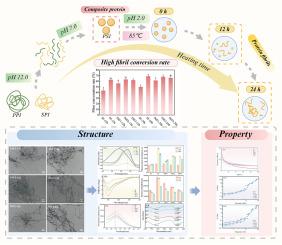
Fibrillation mechanism of soybean protein isolate and pea protein isolate composites: Focused on structure and rheological properties
Plant protein fibrils have significant potential for development in the food industry and have garnered considerable attention in recent years. However, the interactions between heterologous plant proteins during fibril formation remain largely unexplored. In this study, novel composite fibrils (PSF) were fabricated under acidic heating (pH 2.0, 85 °C, 0–24 h) using hybrid proteins prepared through pH-shifting treatment of soy protein isolate and pea protein isolate at varying ratios (1:2, 1:1, 2:1). SPI and PPI exhibited competitive growth mechanism within PSF, with the dominant protein component dictating the fibrillation process. When heated for 12 h, PSF with a SPI to PPI ratio of 1:1 demonstrated the strongest fibrillation capability and optimal rheological properties, achieving a fibril conversion rate exceeding 60 % and a β-sheet content of 45.21 %. Microstructural analysis revealed that increasing the PPI proportion resulted in smoother fibril surfaces (Rq = 1.33 nm), effectively suppressing the surface gelation tendency of soy protein fibrils. FTIR analysis confirmed hydrogen bonding and hydrophobic interactions as the primary driving forces for fibril formation, and the incorporation of PPI enhanced the structural stability of the fibrillar network. Molecular docking validated the binding potential between PPI and SPI, laying the theoretical groundwork for subsequent fibrillation. This study innovatively engineered structurally and functionally tunable plant protein fibrils to broaden their applications in the food industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


