通过水热炭化生产呋喃化合物的云杉树皮的可持续增值
IF 7.1
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究旨在通过水热炭化(HTC)法提取5-羟甲基糠醛(5-HMF)、糠醛(F)和5-甲基糠醛(5-MF) 3种呋喃化合物,对低价值残渣云杉树皮(PB)进行可持续活化。采用响应面法(RSM)和全因子设计(FFD)实验,评价了温度(160 ~ 240℃)、反应时间(1 ~ 24 h)和粒径(50 ~ 1000µm)对呋喃化合物生产的影响。统计分析表明,温度是影响最大的因素,其次是反应时间,而粒径对其影响不显著,尽管组成不同:小粒径的纤维素和木质素含量较多,大粒径的半纤维素和萃取物含量较多。动力学模型证实,5-HMF、F和5-MF是铅衍生糖转化为人蛋白的中间产物。HTC过程中的pH曲线与呋喃化合物的演化相关,最初由于酸的形成而降低,然后随着酸性较低的副产物(可能是酚类物质)的形成而增加。5-HMF的最佳产率为180°C, F和5-MF的最佳产率为160°C,反应时间为7 ~ 8 h。多次优化发现,170°C和9.35 h是同时最大化这三种化合物的最佳条件。这些发现有助于木质纤维素生物质的可持续增值,并证明了HTC在有效回收呋喃化合物方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
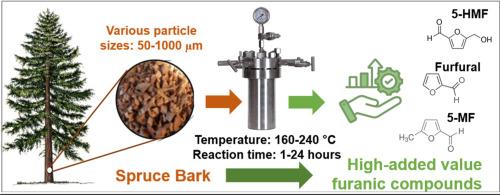
Sustainable valorization of spruce bark via hydrothermal carbonization for the production of furanic compounds
This study aimed to sustainably valorize of Picea abies bark (PB), a low value residue, by extracting three furanic compounds, namely 5-hydroxymethylfurfural (5-HMF), furfural (F) and 5-methylfurfural (5-MF) through hydrothermal carbonization (HTC). Using response surface methodology (RSM) with a full factorial design (FFD) of experiments, the effects of temperature (160-240°C), reaction time (1-24 h) and particle size (50-1000 µm) on furanic compound production were evaluated. Statistical analysis revealed that temperature was the most influential factor, followed by reaction time, while particle size had no significant effect, despite differences in composition: small sizes are richer in cellulose and lignin, and large sizes are richer in hemicelluloses and extractives. Kinetic modeling confirmed that 5-HMF, F, and 5-MF act as intermediates in the transformation of PB-derived sugars to humins. The pH profile during HTC correlated with furanic compound evolution, initially decreasing due to acid formation, then increasing as less acidic byproducts, likely phenolics, formed. Optimal individual yields were obtained at 180°C for 5-HMF and 160°C for both F and 5-MF, with reaction times between 7 and 8 h. Multiple optimizations identified 170°C and 9.35 h as the best conditions for maximizing all three compounds simultaneously. These findings contribute to the sustainable valorization of lignocellulosic biomass and demonstrate the potential of HTC for efficient furanic compound recovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


